Application of metabonomics in regulation study of plant secondary metabolites
-
摘要:
代谢组学是继蛋白质组学和基因组学之后发展起来的新型学科,主要研究生物样本和器官小分子代谢产物,目前广泛应用于生物医学、食品科学、农业动植物学等领域。本文总结了代谢组学技术在植物次生代谢调控研究中的应用,对近5年代谢组学技术在植物热门领域的应用情况进行概括,以期为代谢组学在植物研究领域方面的进一步发展提供理论依据。
Abstract:Metabonomics is a new discipline that has emerged after proteomics and genomics, and is mainly applied to study biological samples and small molecule metabolites in organs. Currently, it is widely used in biomedicine, food science, agricultural zoology, botany, and other fields. This paper summarizes the application of metabolomics technology in the regulation of plant secondary metabolism as well as the research status of metabolomics in popular fields of plant research in the past five years to provide a theoretical basis for the further development of metabolomics research.
-
Keywords:
- Metabolomics /
- Botany /
- Secondary Metabolism /
- Difference
-
地黄(Rehmannia glutinosa Libosch.)为玄参科地黄属植物,多年生草本,以块根入药,为著名的“四大怀药”之一。地黄最早记载见《神农本草经》,被列为上品,迄今已有2000余年的应用历史。根据炮制方法的不同,地黄药材分为鲜地黄、生地黄和熟地黄[1]。2020版《中国药典》记载,鲜地黄具有清热生津、凉血、止血的功效,生地黄具有清热凉血、养阴生津的功效,熟地黄具有补血滋阴,益精填髓的功效[2]。地黄富含环烯醚萜类、苯乙醇苷类、紫罗兰酮类、三萜类、黄酮类和糖类等,对人体心脑血管、血液、中枢神经和免疫系统等均有显著作用[3]。
毛蕊花糖苷(Acteoside)是地黄中含量较高的苯乙醇苷类化合物[4],具有抗氧化、免疫调节、抗炎、保肝、抗肿瘤、增强记忆力等生物活性[5],是2010版、2015版《中国药典》规定的地黄药材质量控制的指标性成分之一。地黄毛蕊花糖苷的含量易受品种[6]、产地[7]、收获时期[5]、种植密度[8]和光照条件[9]等因素的影响,造成某些年份部分地黄药材的毛蕊花糖苷含量达不到《中国药典》规定的要求。地黄毛状根中也含有丰富的毛蕊花糖苷,本课题组前期研究表明,在毛状根诱导的特定时期添加水杨酸(SA)可显著促进毛蕊花糖苷的含量[10]。然而,地黄生长发育过程中叶面喷施SA对毛蕊花糖苷的含量是否有影响还未见报道。
本研究以大田栽培的地黄为材料,采用叶面喷施方法分析SA对地黄叶片和块根毛蕊花糖苷含量的影响,并利用转录组测序技术分析地黄块根中的基因表达特征,研究结果旨在为生产中应用外源激素提高毛蕊花糖苷的含量提供理论依据。
1. 材料与方法
1.1 实验材料
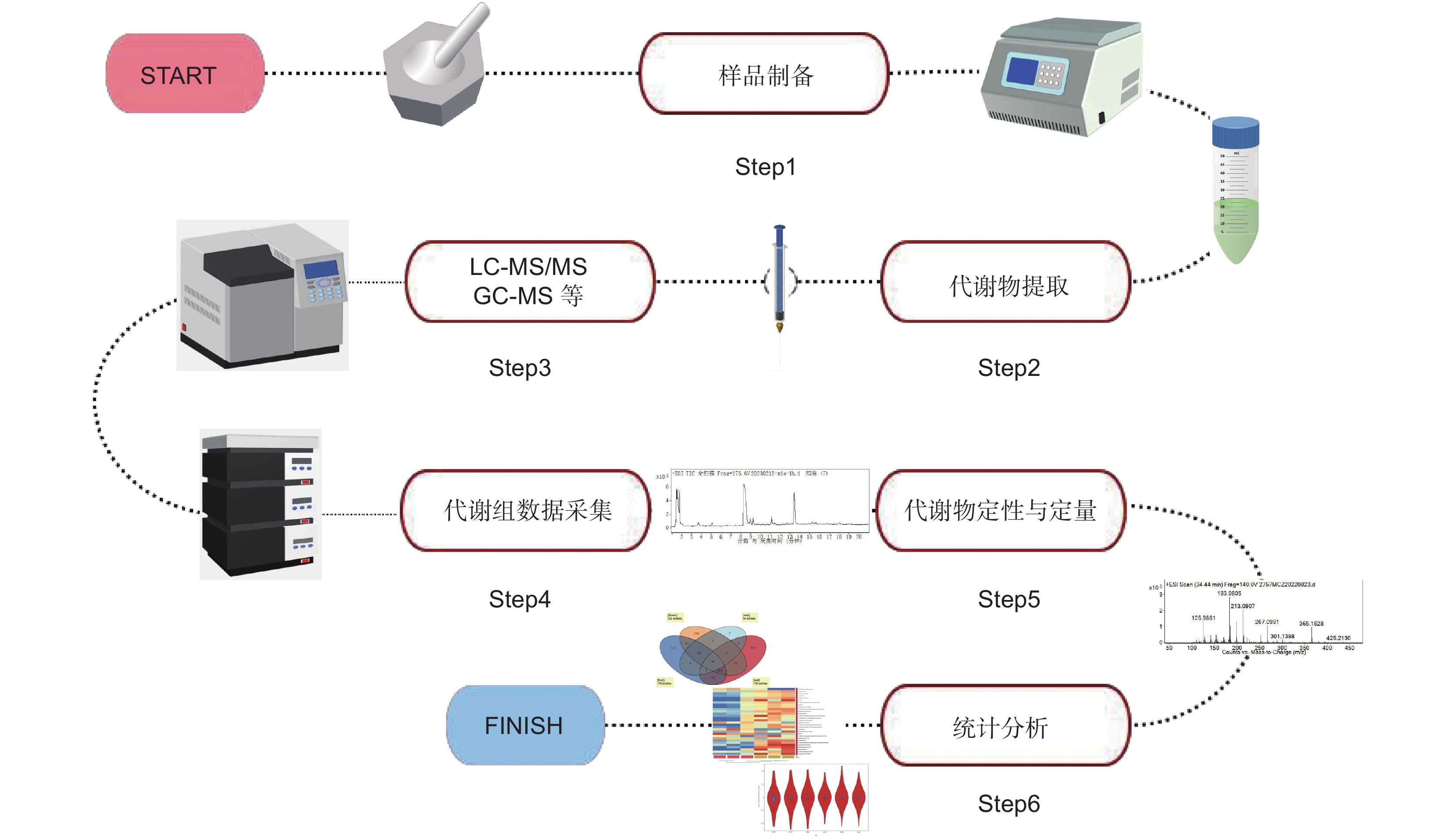
供试材料为地黄‘温85-5’,种植在河南省武陟县蔡庄村(35°2′51″N,113°18′34″E),经河南农业大学王丰青教授鉴定为Rehmannia glutinosa Libosch.。将浓度为100 μmol/L的SA水溶液均匀喷施在生长180 d处于膨大后期长势一致的地黄叶片上,以叶片完全湿润且无水滴落下为准。分别于处理后1、3和6 h进行取样,取样时选择位置相同的叶片。对照组喷施蒸馏水,与处理组同时取样。样品清洗干净后,将块根切成1 cm3左右的小块,叶片剪碎,55℃烘干后打粉,过3号药典筛。样品粉末存放于干净的8号自封袋中备用。
1.2 毛蕊花糖苷含量测定
对照品毛蕊花糖苷(批号MUST-18032725)购于成都曼斯特科技有限公司,纯度均 ≥ 98%。采用 Agilent1260高效液相色谱仪(美国安捷伦科技有限公司)进行检测。
色谱条件:采用的色谱柱型号为 Dikma Diamonsil C18(4.6 × 250 mm,5 μm),柱温30℃,流速1 min/mL。毛蕊花糖苷的流动相为乙腈−0.1%醋酸水(16 : 84),检测波长334 nm,进样量为20 μL。
供试样品溶液制备:精密称取地黄叶和块根样品粉末0.8 g,放入锥形瓶中,精密吸取50 mL甲醇加入锥形瓶中,称重,并在65℃加热回流提取1.5 h,放凉至室温称重,用甲醇补足失重后,摇匀过滤。于蒸发皿中精密吸取滤液20 mL进行毛蕊花糖苷分析,在电热恒温水浴锅上浓缩至近干,残渣用流动相溶解,转移至5 mL容量瓶中,用流动相稀释至刻度,摇晃均匀,用0.22 μm微孔的滤膜过滤,滤液装入2 mL的进样瓶待测。
含量计算:以本实验室建立的标准曲线Y = 30024X − 110.9来计算毛蕊花糖苷含量,Y为峰面积积分值,X为样品的质量浓度。
1.3 样品RNA提取及高通量测序
用TRIzol试剂提取样品的总RNA,用核酸测定仪检测RNA的浓度和质量。使用带有Oligo dT的磁珠富集具有polyA尾巴的mRNA,然后将RNA片段化,反转录后再合成cDNA第2链,形成双链cDNA。双链cDNA经过末端修复、3′末端加A、添加测序接头、多轮扩增、热变性成单链及单链环化等一系列步骤后,完成测序文库的制备。测序委托华大基因科技有限公司进行,测序平台为BGISEQ-500。
对测序得到的原始序列(Raw reads)进行质控处理,去除低质量、接头及污染序列,获取过滤后的测序序列(Clean reads)。使用Bowtie2将Clean reads比对到课题组前期获得的地黄叶和根的参考基因序列集[11],统计不同样品的片段序列比对率以及分布,之后再使用RSEM 计算基因的表达水平,表达量用FPKM表示。对比分析水杨酸喷施前后地黄块根中基因的表达水平,获取水杨酸喷施处理的特异响应基因,并对其进行GO注释和KEGG注释,根据注释结果进行功能分类和KEGG pathway分类,并使用R软件中的phyper函数进行富集分析,获得水杨酸喷施处理下地黄块根内的关键分子响应进程。
1.4 实时荧光定量(qRT-PCR)分析
用TaKaRa反转录试剂盒对RNA进行反转录,合成cDNA,反应体系包括1 μL oligo dT primer,1 μL dNTP Mixture,2 μg 模板RNA,加水补足体积到10 μL,65℃保温5 min后,冰上迅速冷却。再加0.5 μL的RNase抑制剂,1 μL PrimeScript Ⅱ RTase,4 μL 5 × PrimeScript Ⅱ Buffer,加水补足体积到20 μL。反应程序为42℃ 60 min,95℃ 5 min。以RgTIP41为内参基因,用实时荧光定量PCR检测基因表达水平。所用试剂盒为SYBR® Premix Ex Taq™ Ⅱ(Tli RNaseH Plus) (Takara,大连),使用仪器为Bio-Rad IQ5(上海伯乐公司)。定量反应体系为25 μL,包含2 μL 上述反转录cDNA产物,上、下游引物各1 μL,12.5 μL SYBR® Premix Ex Taq,8.5 μL ddH2O。反应程序为:95℃变性30 s;然后95℃ 5 s, 60℃ 30 s,40个循环。结束反应后获得不同样品的扩增循环数Ct,使用2-ΔΔCt法计算不同基因的相对表达量。
2. 结果与分析
2.1 SA处理对地黄毛蕊花糖苷含量的影响
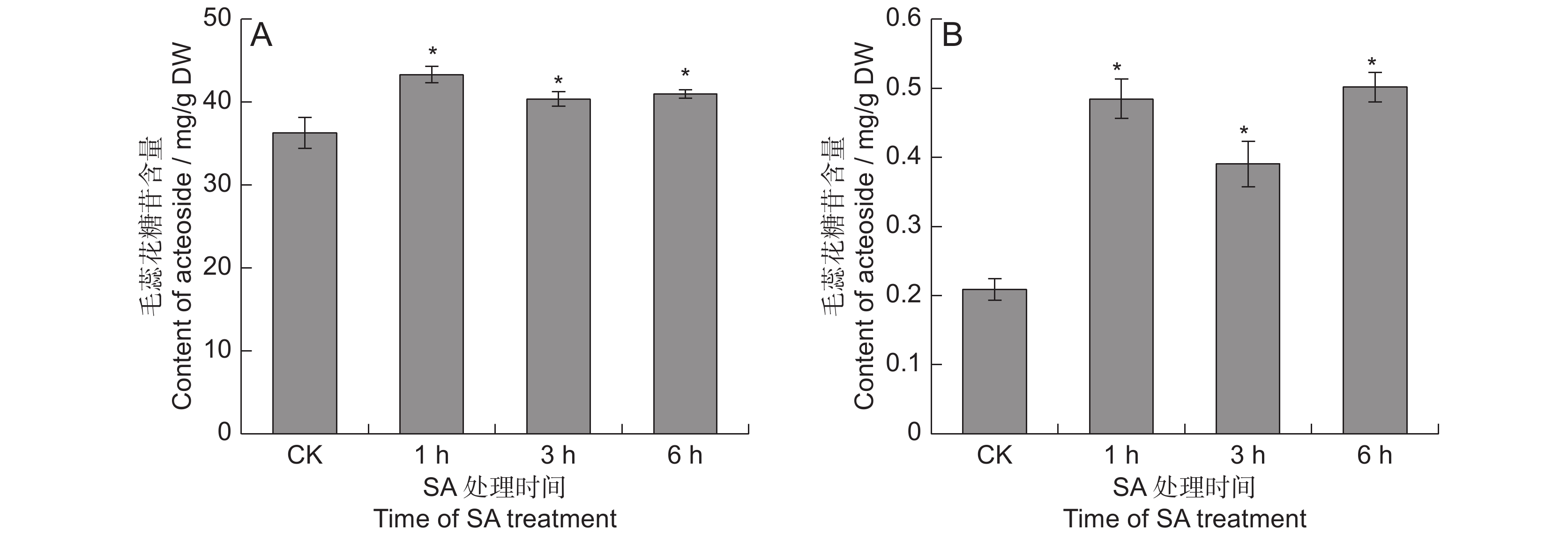
对SA处理的地黄叶和块根中的毛蕊花糖苷含量进行测定,结果表明,SA能够显著提高毛蕊花糖苷的含量(图1)。在叶中, SA处理1 ~ 6 h后,毛蕊花糖苷含量分别提高了11.2% ~ 19.3%。块根中毛蕊花糖苷的提升幅度远高于叶片,分别较对照提高了0.9 ~ 1.4倍,处理 6 h后的毛蕊花糖苷含量最高,达0.5 mg/g,远超2015版《中国药典》规定的0.02%,说明地黄叶面喷施SA可显著提高叶和块根中毛蕊花糖苷的含量。
2.2 地黄块根RNA测序分析
利用Agilent 2100 Bioanalyzer和Fragment Analyzer分别对提取的各样品总RNA质量进行检测,结果显示,12个样品的总RNA浓度在430 ~ 1260 ng/μL,总RNA质量在8.6 ~ 25.2 μg,RNA的浓度和总量满足建库需求。RIN值在8.2 ~ 9.9,28S/18S > 1.6,说明RNA较为完整,符合建库要求。
为了分析SA处理后地黄块根相关基因的表达特性,对SA处理1、3和6 h后的地黄块根进行RNA-seq分析,结果表明(表1),每个测序样本获得的总原始读段量均为21.94 M,去除低质量、接头污染及未知碱基N含量过高的reads,获得的高质量reads在20.98 ~ 21.27 M,碱基数均在1.05 ~ 1.06 Gb,测序数据量基本一致。将每个样品的测序数据匹配地黄参考转录组,发现匹配率在85.04% ~ 87.70%,特异匹配率在47.35% ~ 51.31%,测序数据能够较好地反映细胞中基因表达的真实情况,说明测序质量良好,可以进行后续基因表达分析。
表 1 测序数据统计结果Table 1. Statistics of sequenced data样本
Sample总原始序列
Total raw reads / M总测序序列
Total clean reads / M总测序碱基数
Total clean bases / Gb测序序列比率
Clean read ratio / %总匹配率
Total mapped / %特异匹配率
Uniquely mapped / %Control_1 21.94 21.13 1.06 96.3 87.70 51.31 Control_2 21.94 21.07 1.05 96.04 86.92 51.18 Control_3 21.94 21.08 1.05 96.08 85.72 51.09 SA 1h_1 21.94 21.27 1.06 96.93 85.44 47.35 SA 1h_2 21.94 21.08 1.05 96.05 87.07 48.03 SA 1h_3 21.94 21.15 1.06 96.37 87.28 49.26 SA 3h_1 21.94 20.98 1.05 95.59 86.85 50.36 SA 3h_2 21.94 21.05 1.05 95.93 85.74 49.92 SA 3h_3 21.94 21.05 1.05 95.95 86.81 50.47 SA 6h_1 21.94 21.09 1.05 96.11 86.50 50.25 SA 6h_2 21.94 21.06 1.05 96.00 85.04 49.42 SA6h_3 21.94 21.03 1.05 95.86 86.68 49.97 2.3 SA处理前后差异表达基因分析及筛选
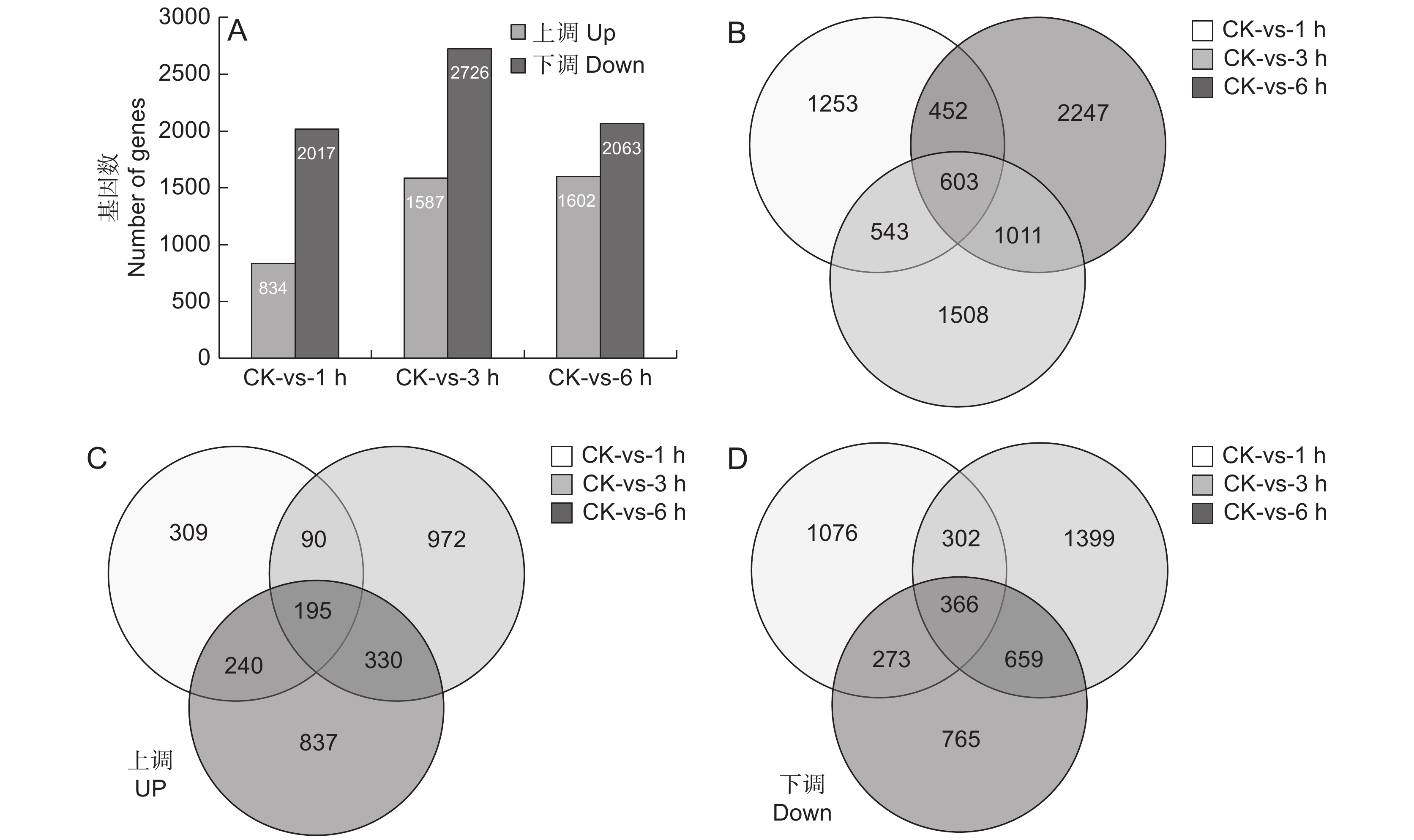
为了分析SA处理后块根中基因的表达特性,采用FPKM值比较基因丰富度的差异(图2)。使用以下标准对差异表达基因(DEGs)进行识别和筛选:校正P值 < 0.001且log2值 ≥ 2。分析SA处理不同时间后与对照样品中的差异表达基因(图2:A),发现SA处理1 h后834个基因上调,2017个基因下调;处理3 h后1587个基因上调,2726个基因下调;处理6 h后1602个基因上调,2063个基因下调。进一步分析SA处理后的3个时间点与CK相比的共同差异表达基因,发现共有603个基因是共同差异表达的(图2:B),其中上调表达和下调表达的基因数分别为195(图2:C)和366个(图2:D)。
![]() 图 2 SA处理后基因显著差异表达A:SA处理过程中上调和下调的基因数目; B ~ D:SA处理后不同时间点鉴别出的总DGEs(B)、上调DGEs(C)和下调DGEs的维恩图。Figure 2. Significant DEGs in response to SA treatmentA: Up-regulated and down-regulated gene numbers during SA treatment; B − D: Venn diagram of total DEGs (B), up-regulated DEGs (C), and down-regulated DEGs (D) identified at different time points after SA treatment.
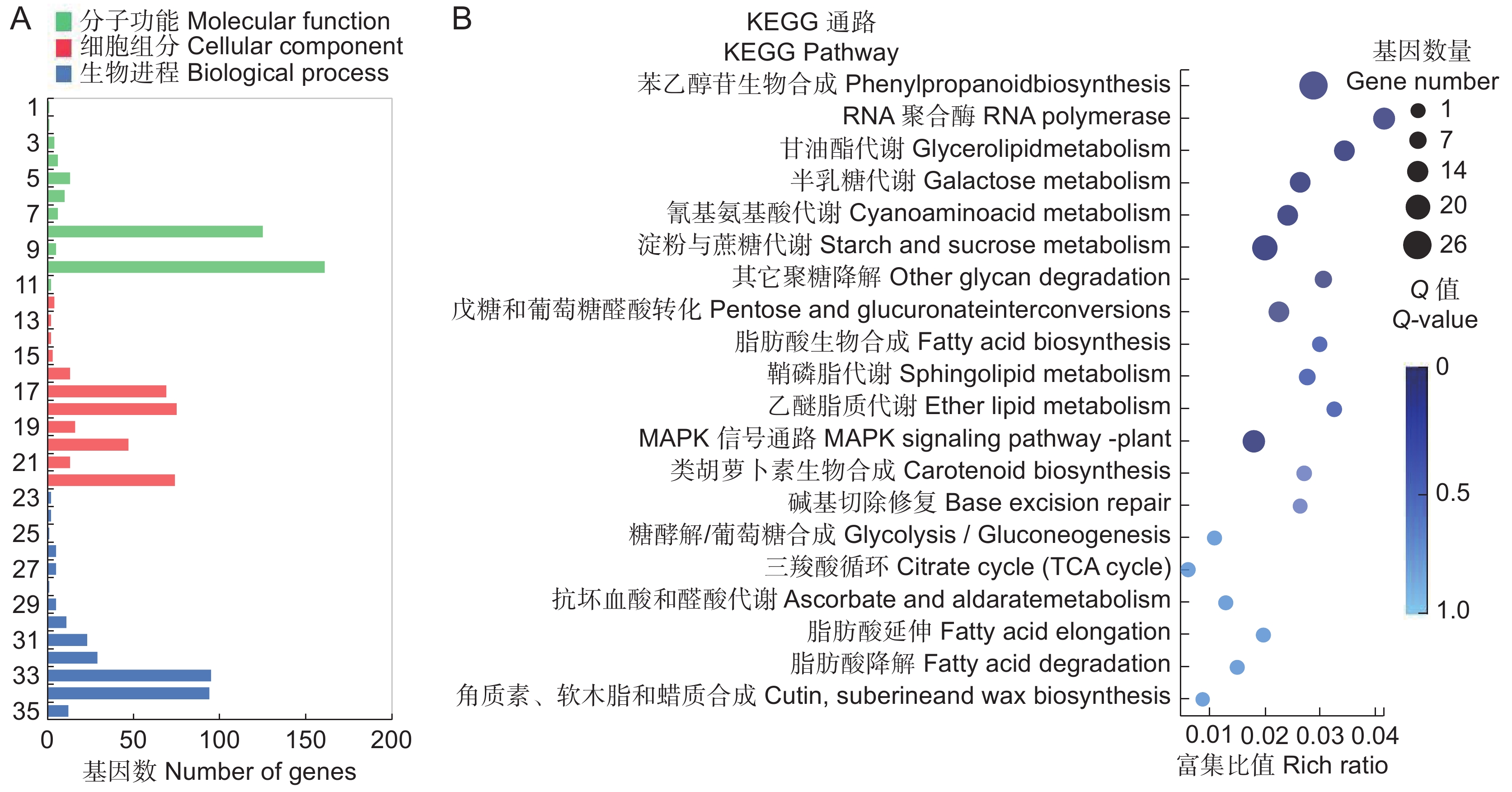
图 2 SA处理后基因显著差异表达A:SA处理过程中上调和下调的基因数目; B ~ D:SA处理后不同时间点鉴别出的总DGEs(B)、上调DGEs(C)和下调DGEs的维恩图。Figure 2. Significant DEGs in response to SA treatmentA: Up-regulated and down-regulated gene numbers during SA treatment; B − D: Venn diagram of total DEGs (B), up-regulated DEGs (C), and down-regulated DEGs (D) identified at different time points after SA treatment.对603个DGEs进行GO功能分类(图3:A),结果显示其共分为分子功能(Molecular function)、细胞功能(Cellar function)和生物功能(Biological function)3个大类。其中分子功能分类中的催化活性(Catalytic activity)占比最多,其次是ATP结合;在细胞功能分类中,细胞(Cell)、细胞膜(Membrane)、细胞膜构件(Membrane part)较多;生物功能分类中,细胞过程(Cellular process)和代谢过程(Metabolic process)所占比重最多。进一步对603个DEGs进行KEGG代谢通路富集分析,图3:B展示了最显著的前20个代谢通路,这些通路涉及各项生命活动。其中首先被富集的是苯乙醇苷生物合成通路(Phenylpropanoid biosynthesis),其次是淀粉和蔗糖代谢合成通路(Starch and sucrose metabolism)、植物MARK信号通路(MAPK signaling pathway plant)及RNA聚合酶通路(RNA polymerase)。这说明SA喷施对地黄块根中次生代谢物的积累产生了较大影响,且调控了碳水化合物和MAPK等多个代谢通路。
![]() 图 3 SA处理后3种比对均差异表达基因的GO分类及KEGG通路富集A:603个基因的GO分类;B:KEGG富集的前20个代谢通路。圆点大小和颜色分别表示通路中DEGs的数量和Q值范围。1:分子转导活性;2:分子载体活性;3:信号转导活性;4:结构分子活性;5:转运活性;6:转录调节活性;7:抗氧化活性;8:结合;9:分子功能调节;10:催化活性;11:膜封闭腔;12:超分子复合物;13:共质体;14:细胞连接;15:细胞组分;16:细胞外区域;17:膜组分;18:膜;19:细胞器组分;20:细胞器;21:大分子复合物;22:细胞;23:生殖过程;24:繁殖;25:解毒作用;26:多细胞生物过程;27:发育过程;28:多生物体过程;29:信号;30:定域化;31:生物调节;32:对刺激的反应;33:代谢过程;34:细胞过程;35:细胞成分组织或生物合成。Figure 3. GO classification and KEGG pathway enrichment of co-DEGs in three comparisonsA: GO classification of 603 genes; B: Top 20 enriched KEGG pathways among 603 genes. Size and color of dot represent number and scope of DEGs in pathway, respectively. 1: Molecular transducer activity; 2: Molecular carrier activity; 3: Signal transducer activity; 4: Structural molecule activity; 5: Transporter activity; 6: Transcription regulator activity; 7: Antioxidant activity; 8: Binding; 9: Molecular function regulator; 10: Catalytic activity; 11: Membrane-enclosed lumen; 12: Supramolecular complex; 13: Symplast; 14: Cell junction; 15: Cell part; 16: Extracellular region; 17: Membrane part; 18: Membrane; 19: Organelle part; 20: Organelle; 21: Macromolecular complex; 22: Cell; 23: Reproductive process; 24: Reproduction; 25: Detoxification; 26: Multicellular organismal process; 27: Developmental process; 28: Multi-organism process; 29: Signaling; 30: Localization; 31: Biological regulation; 32: Response to stimulus; 33: Metabolic process; 34: Cellular process; 35: Cellular component organization or biogenesis.
图 3 SA处理后3种比对均差异表达基因的GO分类及KEGG通路富集A:603个基因的GO分类;B:KEGG富集的前20个代谢通路。圆点大小和颜色分别表示通路中DEGs的数量和Q值范围。1:分子转导活性;2:分子载体活性;3:信号转导活性;4:结构分子活性;5:转运活性;6:转录调节活性;7:抗氧化活性;8:结合;9:分子功能调节;10:催化活性;11:膜封闭腔;12:超分子复合物;13:共质体;14:细胞连接;15:细胞组分;16:细胞外区域;17:膜组分;18:膜;19:细胞器组分;20:细胞器;21:大分子复合物;22:细胞;23:生殖过程;24:繁殖;25:解毒作用;26:多细胞生物过程;27:发育过程;28:多生物体过程;29:信号;30:定域化;31:生物调节;32:对刺激的反应;33:代谢过程;34:细胞过程;35:细胞成分组织或生物合成。Figure 3. GO classification and KEGG pathway enrichment of co-DEGs in three comparisonsA: GO classification of 603 genes; B: Top 20 enriched KEGG pathways among 603 genes. Size and color of dot represent number and scope of DEGs in pathway, respectively. 1: Molecular transducer activity; 2: Molecular carrier activity; 3: Signal transducer activity; 4: Structural molecule activity; 5: Transporter activity; 6: Transcription regulator activity; 7: Antioxidant activity; 8: Binding; 9: Molecular function regulator; 10: Catalytic activity; 11: Membrane-enclosed lumen; 12: Supramolecular complex; 13: Symplast; 14: Cell junction; 15: Cell part; 16: Extracellular region; 17: Membrane part; 18: Membrane; 19: Organelle part; 20: Organelle; 21: Macromolecular complex; 22: Cell; 23: Reproductive process; 24: Reproduction; 25: Detoxification; 26: Multicellular organismal process; 27: Developmental process; 28: Multi-organism process; 29: Signaling; 30: Localization; 31: Biological regulation; 32: Response to stimulus; 33: Metabolic process; 34: Cellular process; 35: Cellular component organization or biogenesis.2.4 毛蕊花糖苷合成相关催化酶基因表达分析
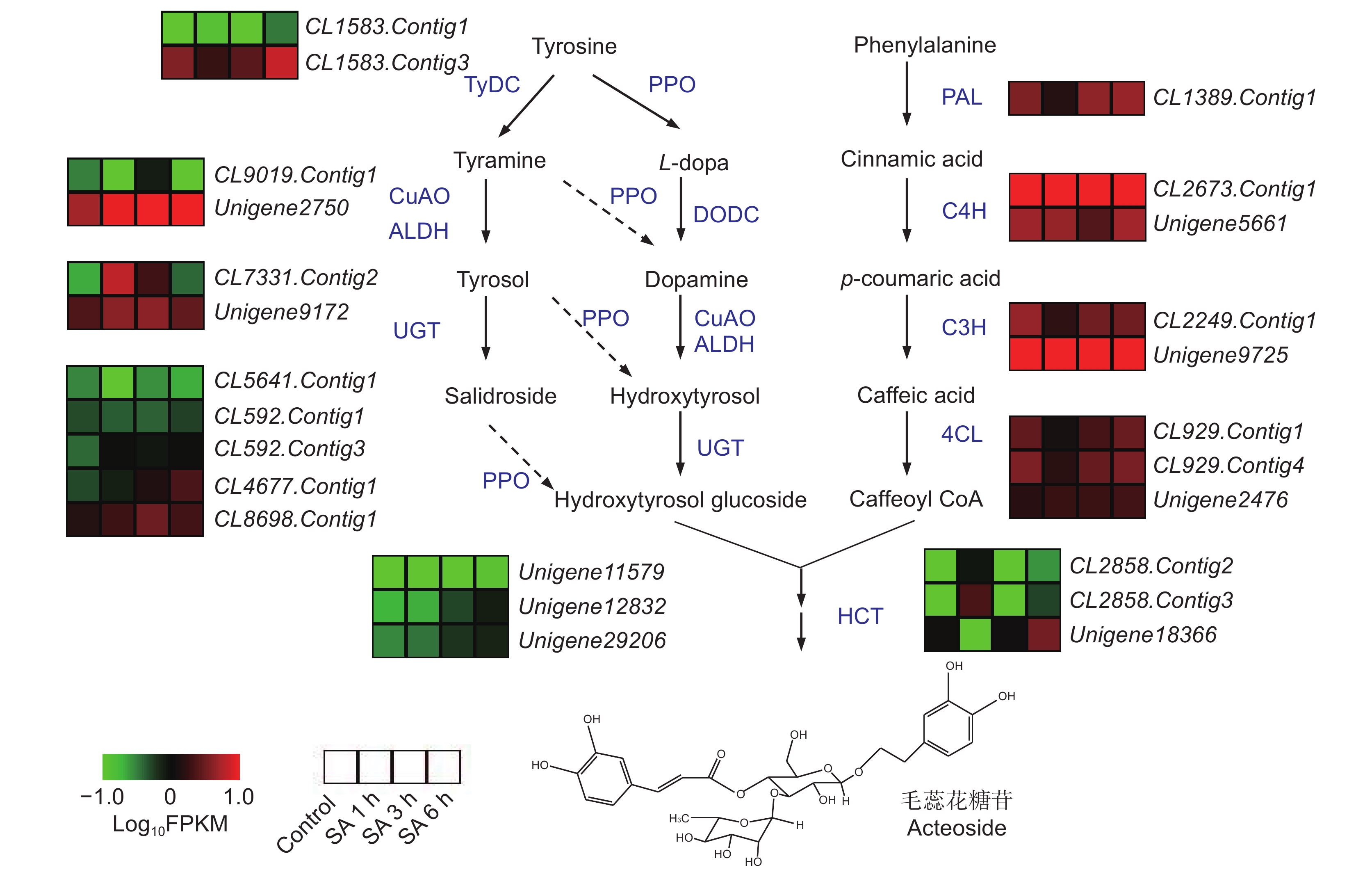
研究表明,在植物体内,毛蕊花糖苷由苯丙氨酸途径的咖啡酰辅酶A(Caffeoyl CoA)和酪氨酸途径的羟基酪醇苷(Hydroxtrosol glucoside)经缩合糖苷化后生成[8]。在地黄转录组中鉴定出可能参与毛蕊花糖苷合成的编码催化酶的基因215个,但SA处理后,地黄块根中仅有少数催化酶基因上调表达(图4)。在酪氨酸途径中,编码乙醛脱氢酶(ALDH)的基因CL7331.Contig2,在SA处理1 h和3 h后的地黄块根中表达量显著升高,另一个ALDH编码基因Unigene9172在SA处理后的表达量也有一定程度上升。编码糖苷转移酶(UGT)的基因CL4677.Contig1随着SA处理时间的延长其表达量逐渐升高,在SA处理6 h后表达量最高。编码多酚氧化酶(PPO)的两个基因Unigene12832和Unigene12832在SA处理3 h和6 h后的表达量增加较为明显。而苯丙氨酸途径的催化酶基因表达变化不明显。这说明SA处理后毛蕊花糖苷含量的增加可能主要与酪氨酸途径部分催化酶基因的表达量增加有关。
2.5 差异表达转录因子筛选
对SA处理不同时间后地黄块根中差异表达的转录因子进行分析,结果表明(表2),25种转录因子的编码基因在处理前后呈差异表达。在处理1和3 h后,下调表达的转录因子较多,处理6 h后则上调表达的转录因子较多。WRKY、MYB、bHLH、AP2-EREBP、NAC和GRAS转录因子的差异表达基因较多,其中AP2-EREBP、WRKY和MYB的差异表达基因最多,且均表现为SA处理1 h后下调的基因较多, 3 h和6 h后上调表达的基因较多。进一步分析发现,共有20个转录因子编码基因在SA处理后的3个时间点均上调表达(表3),其中NAC和AP2-EREBP基因均为4个,WRKY基因有3个,MYB、GRAS、PLATZ基因各2个,bHLH、MADS和C2C2-CO-lik基因各1个。具有调控毛蕊花糖苷合成功能的RgWRKY37编码基因CL394.Contig2在SA处理1、3、6 h后的Log2(SA处理/CK)的值分别为0.79、0.82和0.57,Log2(SA处理/CK)的值虽然小于1,但其Q-value和P-value均达到了显著水平,说明在SA处理后CL394.Contig2上调表达。
表 2 SA处理后差异表达的转录因子数Table 2. Number of differentially expressed transcription factors (TFs) after SA treatment转录因子
Transcription factorCK-vs-1 h CK-vs-3 h CK-vs-6 h 共同差异表达的基因数
Number of common DEGs下调 Down 上调 Up 下调 Down 上调 Up 下调 Down 上调 Up zf-HD 2 1 1 1 0 0 0 WRKY 11 5 8 16 5 15 5 TUB 1 0 1 1 0 0 0 Trihelix 1 0 1 0 0 0 0 Tify 1 0 3 0 3 0 1 PLATZ 0 4 0 4 1 3 2 SRS 0 0 2 0 2 0 0 OFP 2 0 3 0 1 0 0 NAC 2 8 2 6 0 8 4 MYB 15 10 5 7 6 12 3 mTERF 1 0 2 0 1 1 0 MADS 2 2 3 4 2 2 1 LOB 1 0 4 2 2 0 0 HSF 5 2 4 6 3 6 2 GRAS 1 8 0 4 0 7 2 G2-like 2 0 2 1 0 3 0 CPP 2 0 2 0 4 0 2 C2H2 3 1 8 1 3 1 2 C2C2-GATA 2 0 1 0 5 0 0 C2C2-Dof 1 1 3 0 2 1 0 C2C2-CO-like 0 1 0 2 4 3 1 bZIP 1 2 0 0 1 0 0 bHLH 12 3 15 4 5 4 2 AP2-EREBP 15 12 12 18 9 21 6 ABI3VP1 5 2 7 1 1 1 0 总数 88 62 89 78 60 88 33 表 3 SA处理后上调表达的转录因子基因Table 3. Up-regulated transcription factor genes after SA treatment转录因子
Transcription factor基因
GeneLog2(SA/CK) 功能
Function1 h 3 h 6 h AP2-EREBP CL1637.Contig3 2.82 1.29 2.42 Ethylene-responsive transcription factor ERF071 AP2-EREBP CL4501.Contig2 1.66 1.75 2.16 Pathogenesis-related genes transcriptional activator PTI6 AP2-EREBP CL7827.Contig1 1.77 3.51 4.24 Ethylene-responsive transcription factor ERF106-like AP2-EREBP Unigene2558 1.62 1.06 1.74 Ethylene-responsive transcription factor 2-like MYB CL1983.Contig1 4.55 4.19 3.12 Transcription factor TFIIIB component B''-like MYB CL4303.Contig1 3.76 2.61 3.47 Single MYB histone protein NAC CL2945.Contig2 6.82 7.15 7.51 NAC domain-containing protein 82-like isoform X1 NAC CL4851.Contig1 3.15 1.99 2.44 NAC transcription factor 29 NAC CL4851.Contig2 3.15 2.27 2.82 NAC transcription factor 29 NAC Unigene5193 2.09 1.38 2.37 NAC domain-containing protein 72 C2C2-CO-like CL5505.Contig3 2.94 2.66 4.31 Zinc finger protein CONSTANS-LIKE 4-like PLATZ CL5569.Contig1 2.31 1.49 2.53 Interleukin-1 receptor-associated kinase 4 PLATZ CL5569.Contig3 2.47 1.71 2.38 Interleukin-1 receptor-associated kinase 4 GRAS CL645.Contig2 1.93 1.65 2.91 Scarecrow-like protein 14 GRAS Unigene10453 1.03 1.03 1.08 Scarecrow-like protein 15 WRKY CL6521.Contig1 2.84 1.66 2.35 Probable WRKY transcription factor 25 WRKY CL7324.Contig3 1.13 1.59 1.21 Probable WRKY transcription factor 35 WRKY CL791.Contig6 2.59 2.28 2.29 Probable WRKY transcription factor 40 bHLH Unigene12420 2.20 2.20 2.29 Phytochrome-interacting factor 3 MADS Unigene23315 2.17 3.01 2.27 MADS-box transcription factor 2.6 qRT-PCR验证基因表达差异
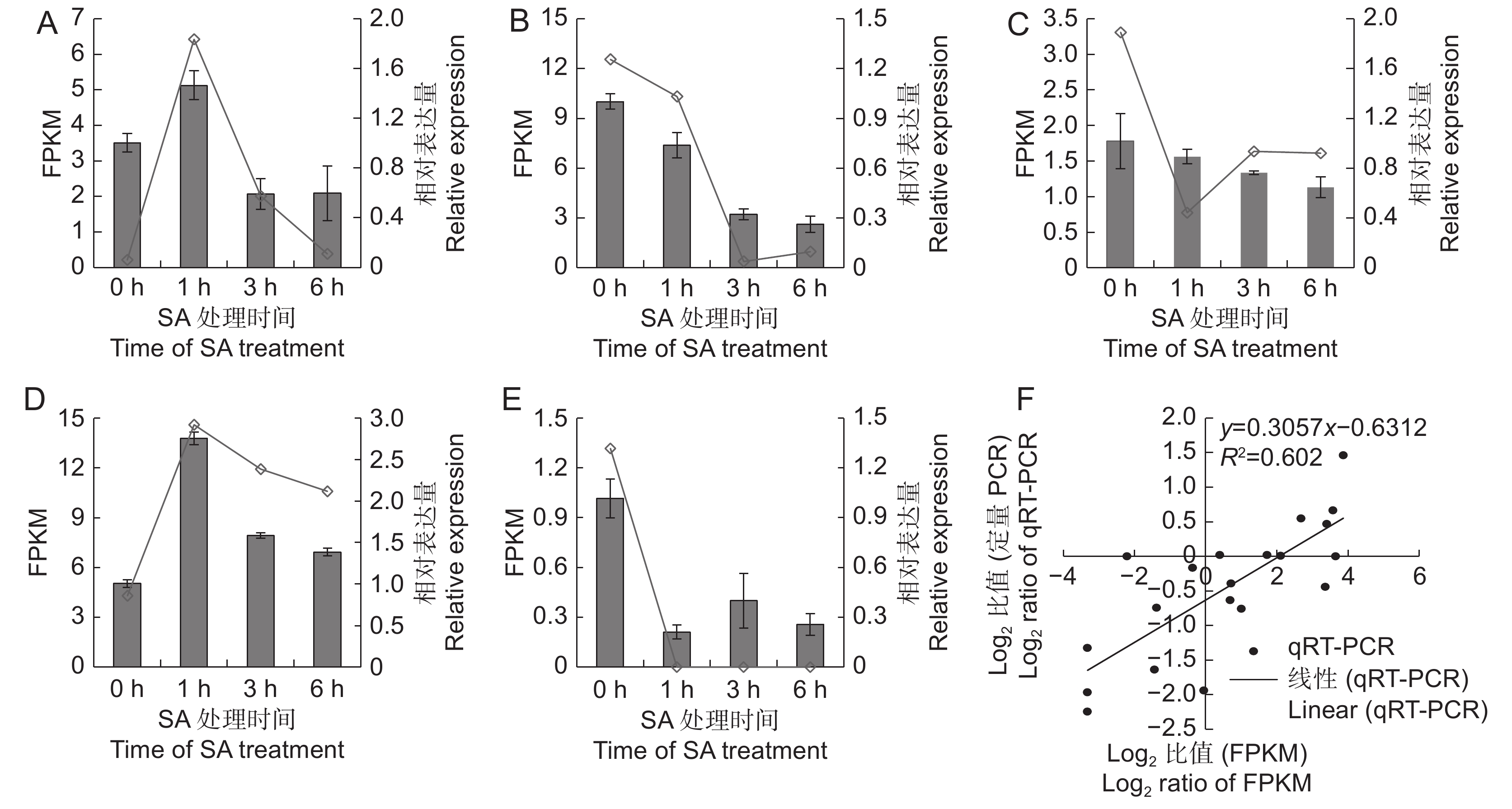
为了验证转录组测序对基因表达量分析的可靠性,随机选取CL7331.Contig2、Unigene1886、CL985.Contig1、CL5931.Contig2和CL379.Contig3等5个基因,利用qRT-PCR方法检测其在不同样本中的相对表达量。结果表明(图5),5个基因的定量结果与转录组获得的FPKM值变化趋势基本一致,其中CL7331.Contig2和CL5931.Contig2为SA处理后上调表达的基因,Unigene1886、CL985.Contig1和CL379.Contig3为SA处理后下调表达的基因。qRT-PCR与转录组测序的Pearson相关系数为0.602,相关性达显著水平,说明利用转录组测序分析SA处理后的基因表达量结果可靠。
![]() 图 5 差异表达基因的qRT-PCR验证A ~ E分别为CL7331.Contig2、Unigene1886、CL985.Contig1、CL5931.Contig2和CL379.Contig3的FPKM值与相对表达量;F:FPKM值与qRT-PCR的相关性分析。Figure 5. Validation of DEGs by qRT-PCRA–E: Represent expression and FPKM values of CL7331.Contig2, Unigene1886, CL985.Contig1, CL5931.Contig2, and CL379.Contig3; F: Correlation analysis between FPKM and qRT-PCR data.
图 5 差异表达基因的qRT-PCR验证A ~ E分别为CL7331.Contig2、Unigene1886、CL985.Contig1、CL5931.Contig2和CL379.Contig3的FPKM值与相对表达量;F:FPKM值与qRT-PCR的相关性分析。Figure 5. Validation of DEGs by qRT-PCRA–E: Represent expression and FPKM values of CL7331.Contig2, Unigene1886, CL985.Contig1, CL5931.Contig2, and CL379.Contig3; F: Correlation analysis between FPKM and qRT-PCR data.3. 讨论
植物次生代谢产物的积累既受自身遗传因素的控制,也受生长过程中生物与非生物环境条件的影响。一般而言,若药用植物的次生代谢产物在细胞中的含量相对较低,则会制约次生代谢产物的最终含量。近年来,人们常利用生物和非生物诱导子对植物进行处理,以提高植物特定次生代谢产物的生产[12]。如花生四烯酸(AA)、SA、茉莉酸甲酯(MeJA)和AgNO3均能够提高紫杉醇的含量[13]。50 µmol/L的乙烯利可以显著促进人参(Panax ginseng C. A. Meyer)根的生长和人参皂苷的积累[14]。诱导子提高苯乙醇苷含量的研究亦有报道,如外源添加Ag + 和腐胺均可以提高肉苁蓉(Cistanche deserticola Y. C. Ma)细胞培养物中松果菊苷和毛蕊花糖苷的含量[15]。本课题组前期研究发现,在地黄毛状根培养的培养基中添加25 μmol/L的SA可将毛蕊花糖苷的含量提高2.28倍[10]。本研究发现,叶面喷施100 μmol/L的SA可显著提高地黄叶片和块根中毛蕊花糖苷的含量,在块根中最高可提高1.4倍,说明在大田中叶面喷施诱导子可有效提高地黄块根中的次生代谢产物,有助于改善中药材的品质。
转录组测序分析不但可以高通量地获得基因表达的有关信息,还能够揭示基因表达与生命现象之间的内在联系,从而表征生命体的生理活动规律并确定其代谢特征[16]。目前,转录组测序不仅用于模式植物和大田作物生长发育及逆境胁迫响应关键基因的筛选[17-19],在药用植物次生代谢产物合成调控的结构基因和转录因子基因的挖掘中也有广泛应用[20-22]。由于地黄为同源四倍体物种,其基因组测序虽有报道[23],但作为参考基因组仍存在一些问题。因此,本研究利用课题组前期获得的地黄根、叶转录组为参考基因集进行分析,发现测序数据的特异匹配率偏低(50%左右),与地黄毛状根转录组测序的结果[10]类似,可能与其为同源四倍体物种有关。地黄叶片表面喷施SA后,上调表达的基因数少于下调表达,与SA处理的地黄毛状根结果[10]不同,这可能与本研究以大田地黄材料进行SA处理有关。本研究还发现,利用RNA-seq分析基因表达与qRT-PCR分析的结果相关系数仅为0.602,虽然达到显著相关,但未达到极显著相关水平,可能与地黄的基因组较大(约2.6 Gb),而采用RNA-seq测序获得的数据量较小有关。因此,对于基因组较大的物种,建议提高RNA-seq测序的深度,以获得更多的基因表达信息,提高基因表达量分析的准确性。
毛蕊花糖苷的生物合成途径目前已经比较清楚,其羟基酪醇基团来源于酪氨酸途径,咖啡酰基团来源于苯丙氨酸途径[24]。本课题组进一步推导、优化了毛蕊花糖苷的生物合成途径,认为毛蕊花糖苷是由羟基酪醇苷和咖啡酰辅酶A在莽草酸邻羟基肉桂酰转移酶(HCT)/毛蕊花糖苷合酶(AcS)和UGT的催化下合成[10]。周延清等[25] 基于地黄代谢组学分析获得了KEGG途径中的香豆酸-3-羟化酶(C3H),并克隆了其全长编码序列。李欣容等[26]根据SA处理下毛状根中催化酶基因的表达特性,鉴定并克隆了响应SA诱导的毛蕊花糖苷合酶基因RgAcS1。Yang等[27] 鉴定了4个酪氨酸脱羧酶(TyDC)基因,遗传转化发现过量表达RgTyDC2和RgTyDC4的地黄块根、纤维根、茎、嫩叶和成熟叶中的毛蕊花糖苷含量均显著高于野生型。Wang等[28]筛选了1个响应SA和H2O2诱导的WRKY转录因子基因RgWRKY37,功能研究发现RgWRKY37过量表达的毛状根转化体中毛蕊花糖苷和总苯乙醇苷的含量均显著高于对照。本研究发现,在SA处理的地黄块根中2个ALDH基因、1个UGT基因和2个PPO基因均上调表达,可能与块根中毛蕊花糖苷的含量增加有关。同时,在SA处理后的3个时间点,编码WKRY、NAC和AP2-EREBP等转录因子的20个基因均显著上调表达,其中RgWRKY37的表达量均明显增加。本研究结果为进一步探讨SA诱导毛蕊花糖苷合成的分子机理奠定了基础。
1 1)如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。 -
表 1 植物生物胁迫代谢组研究列表
Table 1 List of metabolomics studies related to plant disease control
物种
Species侵染病害
Infectious disease相关代谢物
Related metabolites代谢组学分析
Metabolomics analysis参考文献
Reference核桃
Juglans regia L.胶孢炭疽菌 花青素B1/2/3、咖啡酸、没食子酸、柚皮素等 探讨了炭疽菌的发生机制 [16] 红枣
Ziziphus jujuba Mill.链格孢菌、细极链格孢菌 链格孢酚、细交链孢菌酮酸;天冬氨酸、甲硫氨酸和赖氨酸 阐释了黑斑病变过程中链格孢毒素的产生机理 [17] 柑橘
Citrus reticulata Blanco柑橘绿霉菌 糖类、脂质、有机酸 揭示柑橘采后绿脓杆菌的侵染机制 [18] 藜麦
Chenopodium quinoa Willd.霜霉病菌 蔗糖、生物胺、天冬氨酸、ɣ-氨基丁酸等氨基酸 揭示了毒素产生机理 [20] 小麦
Triticum aestivum L.禾谷镰刀菌 氨基酸 优化代谢物提取方法,探究染菌发生机制 [21] 小麦
Triticum aestivum L.腥黑粉菌 葫芦酸、十八碳三烯酸 揭示了籽粒腥黑穗病菌侵染前后代谢谱差异 [22] 柑橘
Citrus reticulata Blanco黄龙病 氨基酸、植物激素、嘌呤、水杨酸 证明了耐黄龙病品种的抗性策略 [19] 人参
Panax ginseng C. A. Meyer人参锈根症状 木质素、脂质、生物碱 揭示了病害的潜在分子机制 [23] 葡萄
Vitis vinifera L.中华蚱蜢、灰葡萄孢菌 脯氨酸等氨基酸、天冬氨酰-L、谷胱甘肽和一些脂肪酸 揭示了代谢组水平的复杂网络调控 [24] 表 2 植物非生物胁迫代谢组研究列表
Table 2 List of metabolomics studies related to plant abiotic stress
物种
Species非生物胁迫因素
Abiotic stress factors相关代谢物
Related metabolites代谢组学分析
Metabolomics analysis参考文献
Reference蓝莓 Vaccinium spp. 盐 甘氨酸、苹果酸、十八烷酸、L-苏糖酸等 耐盐性机理 [26] 水稻 Oryza sativa L. 铅 脂质、类二十烷酸 根际微环境与重金属胁迫耐受性 [25] 白刺 Nitraria tangutorum Bobr. 盐 氨基酸、糖、脂肪酸 白刺对盐胁迫的代谢响应 [27] 党参 Codonopsis pilosula (Franch.) Nannf. 干旱 脂类、糖类 干旱胁迫后产物 [29] 黑麦草 Lolium perenne L. 盐 糖类、氨基酸及其中间代谢物 耐盐代谢通路 [28] 早熟禾 Poa annua L. 低温 糖和糖醇、氨基酸、有机酸 适应低温的主要机制 [30] 橡胶树 Hevea brasiliensis (Willd. ex A. Juss.)Muell. 低温 糖类、氨基酸、脂质类 低温胁迫代谢物响应机制 [31] -
[1] Nicholson JK,Lindon JC,Holmes E. 'Metabonomics':understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data[J]. Xenobiotica,1999,29 (11):1181−1189. doi: 10.1080/004982599238047
[2] Fiehn O. Combining genomics,metabolome analysis,and biochemical modelling to understand metabolic networks[J]. Int J Genom,2001,2 (3):155−168.
[3] Fiehn O,Kopka J,Dormann P,Altmann T,Trethewey RN,Willmitzer L. Metabolite profiling for plant functional genomics[J]. Nat Biotechnol,2000,18 (11):1157−1161. doi: 10.1038/81137
[4] 姚玲玲,柯昌强,刘佳,唐春萍,叶阳. 不同炮制程度中药饮片蜜炙甘草的次生代谢化学成分组学研究[J]. 药学学报,2021,56(5):1444−1452. Yao LL,Ke CQ,Liu J,Tang CP,Ye Y. Metabolomic investigation of secondary metabolites of prepared slices of Glycyrrhiza uralensis with different degrees of honey processing[J]. Acta Pharmaceutica Sinica,2021,56 (5):1444−1452.
[5] 王鑫梦,唐小涵,李盈瑶,普雪雪,周燕. 基于代谢组学与网络药理学分析牛筋果叶中潜在药用活性成分及机制研究[J]. 中国中药杂志,2021,46(14):3625−3632. Wang XM,Tang XH,Li YY,Pu XX,Zhou Y. An integrative metabolomics and network pharmacology method for exploring bioactive components and preliminary pharmacodynamics in medicinal parts of Harrisonia perforata[J]. China Journal of Chinese Materia Medica,2021,46 (14):3625−3632.
[6] 杨靖,许振鹏,刘艳,康立欣,杨炳友,匡海学. 基于代谢组学的补阴药药性研究[J]. 中草药,2021,52(10):2987−2995. Yang J,Xu ZP,Liu Y,Kang LX,Yang BY,Kuang HX. Research on property of Yin-tonifying herbs based on metabolomics[J]. Chinese Traditional and Herbal Drugs,2021,52 (10):2987−2995.
[7] Jang C,Chen L,Rabinowitz JD. Metabolomics and isotope tracing[J]. Cell,2018,173 (4):822−837. doi: 10.1016/j.cell.2018.03.055
[8] Van de Velde B,Guillarme D,Kohler I. Supercritical fluid chromatography - Mass spectrometry in metabolomics:past,present,and future perspectives[J]. J Chromatogr B,2020,1161:122444. doi: 10.1016/j.jchromb.2020.122444
[9] 戴宇樵,吕才有. 代谢组学技术在茶学中的应用研究进展[J]. 江苏农业科学,2019,47(2):24−28. Dai YQ,Lü YC. Research progress of application of metabolomics technology in tea science[J]. Jiangsu Agricultural Sciences,2019,47 (2):24−28.
[10] Sengupta A,Weljie AM. NMR Spectroscopy-Based metabolic profiling of biospecimens[J]. Curr Protoc Protein Sci,2019,98 (1):e98.
[11] Rinschen MM,Ivanisevic J,Giera M,Siuzdak G. Identification of bioactive metabolites using activity metabolomics[J]. Nat Rev Mol Cell Biol,2019,20 (6):353−367. doi: 10.1038/s41580-019-0108-4
[12] 张丽媛,代安娜,于润众,阮长青,李志江,张东杰. 基于代谢组学的黑龙江省不同产地大豆的代谢产物分析[J]. 现代食品科技,2021,37(6):287−295. Zhang LY,Dai AN,Yu RZ,Ruan CQ,Li ZJ,Zhang DJ. Analysis of metabolites of soybeans from different producing origins in Heilongjiang Province Based on metabonomics[J]. Modern Food Science and Technology,2021,37 (6):287−295.
[13] 周曼丽,俞赟丰,罗晓欣,兰晓栋,金梦雨,简维雄. 基于代谢组学技术的冠心病血瘀证不同进展阶段大鼠潜在生物标志物及代谢通路研究[J]. 中国中医药信息杂志,2022,29(1):73−79. Zhou ML,Yu YF,Luo XX,Lan XD,Jin MY,Jian WX. Study on potential biomarkers and metabolic pathways of rats in different progressive stages of coronary heart disease with blood stasis syndrome based on metabonomics technology[J]. Chinese Journal of Information on Traditional Chinese Medicine,2022,29 (1):73−79.
[14] Abdelhafez OH,Othman EM,Fahim JR,Desoukey SY,Pimentel-Elardo SM,et al. Metabolomics analysis and biological investigation of three Malvaceae plants[J]. Phytochem Anal,2020,31 (2):204−214. doi: 10.1002/pca.2883
[15] 赵利,钞建宾,郭捷,田海娇,高芬. 基于代谢组学技术的植物抗病相关代谢物研究进展[J]. 西北植物学报,2021,41(6):1071−1078. Zhao L,Chao JB,Guo J,Tian HJ,Gao F. Study on plant Resistance-related metabolites against pathogenic fungi based on metabolomics[J]. Acta Botanica Boreali-Occidentalia Sinica,2021,41 (6):1071−1078.
[16] 陈新,王敏,傅茂润,王贵芳,相昆,等. 核桃炭疽病发生相关的酚类物质代谢分析[J]. 林业科学,2021,57(10):71−80. Chen X,Wang M,Fu MR,Wang GF,Xiang K,et al. Metabolic analysis of phenolic compounds associated with walnut anthracnose[J]. Scientia Silvae Sinicae,2021,57 (10):71−80.
[17] 范盈盈,胡东强,张锐利,李晓龙,何伟忠,等. 黑斑病对新疆红枣营养成分的影响[J]. 食品科学,2020,41(8):303−307. Fan YY,Hu DQ,Zhang RL,Li XL,He WZ,et al. Effects of black spot disease on nutritional composition of red jujubes grown in Xinjiang[J]. Food Science,2020,41 (8):303−307.
[18] Yang QY,Qian X,Routledge MN,Wu XY,Shi Y,et al. Metabonomics analysis of postharvest citrus response to Penicillium digitatum infection[J]. LWT-Food Sci Technol,2021,152:112371. doi: 10.1016/j.lwt.2021.112371
[19] Suh JH,Tang XX,Zhang Y,Gmitter FG Jr,Wang Y. Metabolomic analysis provides new insight into tolerance of Huanglongbing in citrus[J]. Front Plant Sci,2021,12:710598. doi: 10.3389/fpls.2021.710598
[20] 赵丽娟,闫素月,史晓晶,尉俊海,张洪. 霜霉病菌侵染对藜麦叶片代谢的影响[J]. 植物病理学报,2021,51(3):334−339. Zhao LJ,Yan SY,Shi XJ,Wei JH,Zhang H. Downy mildew-infection changes the metabolism of quinoa leaves[J]. Acta Phytopathologica Sinica,2021,51 (3):334−339.
[21] Zhao PY,Gu SB,Han C,Lu YR,Ma CY,et al. Targeted and untargeted metabolomics profiling of wheat reveals amino acids increase resistance to Fusarium head blight[J]. Front Plant Sci,2021,12:762605. doi: 10.3389/fpls.2021.762605
[22] Ren ZY,Fang MK,Muhae-Ud-Din G,Gao HF,Yang YZ,et al. Metabolomics analysis of grains of wheat infected and noninfected with Tilletia controversa Kühn[J]. Sci Rep,2021,11 (1):18876−18876. doi: 10.1038/s41598-021-98283-3
[23] Bian XB,Zhao Y,Xiao SY,Yang H,Han YZ,Zhang LX. Metabolome and transcriptome analysis reveals the molecular profiles underlying the ginseng response to rusty root symptoms[J]. BMC Plant Biol,2021,21 (1):215. doi: 10.1186/s12870-021-03001-w
[24] Jia HR,Li T,Haider MS,Sadeghnezhad E,Pang QQ,et al. Comparative transcriptomic and metabolomic profiling of grapevine leaves (cv. Kyoho) upon infestation of grasshopper and Botrytis cinerea[J]. Plant Mol Biol Rep,2022,40 (3):539−555. doi: 10.1007/s11105-022-01336-8
[25] Tan B,Tan X,Liu C,Zeng Y,Li YH. Effects of lead stress on rice (Oryza sativa L. ) growth and metabolism in the rhizosphere microenvironment:the role of eicosanoid compounds[J]. Plant Growth Regul,2022,96 (3):483−495. doi: 10.1007/s10725-022-00802-3
[26] 高龙飞,贾斌,张卫华,李爱,李天杰,等. 盐胁迫下蓝莓叶片生理特性与代谢组学分析[J]. 植物生理学报,2022,58(1):155−164. Gao LF,Jia B,Zhang WH,Li A,Li TJ,et al. Physiological characteristics and metabonomics analysis of blueberry leaves under salt stress[J]. Plant Physiology Journal,2022,58 (1):155−164.
[27] 闫海冰,张慧芳,冯帆,于兆友,杨秀清. 2种白刺对盐胁迫的代谢响应机制[J]. 林业科学,2021,57(1):20−29. Yan HB,Zhang HF,Feng F,Yu ZY,Yang XQ. Metabolic response mechanism of two Nitraria species to salt stress[J]. Scientia Silvae Sinicae,2021,57 (1):20−29.
[28] 张彩峡,吴洪新,毕玉芬. NaCl胁迫下多年生黑麦草的代谢组学分析[J]. 中国草地学报,2020,42(4):62−72. Zhang CX,Wu HX,Bi YF. Metabonomics analysis of Lolium perenne under NaCl stress[J]. Chinese Journal of Grassland,2020,42 (4):62−72.
[29] 赵春旭,张然,牛奎举,朱瑞婷,王勇,等. 青海野生草地早熟禾响应低温胁迫的代谢组学研究[J]. 草地学报,2020,28(4):904−914. Zhao CX,Zhang R,Niu KJ,Zhu RT,Wang Y,et al. Metabonomics study of Qinghai wild Poa pratensis in response to low temperature stress[J]. Acta Agrestia Sinica,2020,28 (4):904−914.
[30] 毛常丽, 李玲, 杨湉, 李小琴, 张凤良, 等. 橡胶树‘云研77-4’无性系幼苗低温胁迫后的代谢组学分析[J/OL]. 分子植物育种, 2022: 1−16. (2022-03-24). http: //kns.cnki.net/kcms/detail/46.1068.S.20220322.2041.012.html. Mao CL, Li L, Yang T, Li XQ, Zhang FL, et al. The metabolomics analysis of the clone cultivar 'Yunyan 77-4' clone seedlings under low temperature stress[J/OL]. Molecular Plant Breeding, 2022: 1−16. (2022-03-24). http: //kns.cnki.net/kcms/detail/46.1068.S.20220322.2041.012.html.
[31] 原静静,孙晓琛,栗锦鹏,杜弢,王惠珍. 基于LC-MS的干旱胁迫下党参代谢组学分析[J]. 中国实验方剂学杂志,2021,27(23):145−152. Yuan JJ,Sun XC,Li JP,Du T,Wang HZ. Metabolomics analysis of Codonopsis pilosula under drought stress based on LC-MS[J]. Chinese Journal of Experimental Traditional Medical Formulae,2021,27 (23):145−152.
[32] Xie HM,Wang HD,Chen BX,Lou J,Wang HM,et al. Untargeted metabolomics analysis to unveil the chemical markers for the differentiation among three Gleditsia sinensis-derived herbal medicines by ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry[J]. Arabian J Chem,2022,15 (5):103762. doi: 10.1016/j.arabjc.2022.103762
[33] Liu RY,Deng J,Lin XL,Li YM,Lin Y,et al. Metabolomics reveals distinct metabolites between Lonicera japonica and Lonicera macranthoides based on GC-MS[J]. J Chem,2020,2020:6738571.
[34] Tang YC,Liu YJ,He GR,Cao YW,Bi MM,et al. Comprehensive analysis of secondary metabolites in the extracts from different lily bulbs and their antioxidant ability[J]. Antioxidants,2021,10 (10):1634. doi: 10.3390/antiox10101634
[35] Xia ZD,Liu X,Tong LG,Wang H,Feng ML,et al. Comparison of chemical constituents of Bupleurum marginatum var. stenophyllum and Bupleurum chinense DC. using UHPLC-Q-TOF-MS based on a metabonomics approach[J]. Biomed Chromatogr,2021,35 (9):e5133.
[36] Wang SQ,Li WN,Zhang XF,Li G,Li XD,et al. Metabolomics study of different germplasm resources for three polygonatum species using UPLC-Q-TOF-MS/MS[J]. Front Plant Sci,2022,13:826902. doi: 10.3389/fpls.2022.826902
[37] He YF,Cai HQ,Zhang HM,Ren ZH,Tang H,et al. A metabolomic study of Asian and American ginseng based on RRLC-QTOF/MS methods[J]. J Liq Chromatogr Relat Technol,2019,42 (13-14):452−458. doi: 10.1080/10826076.2019.1625371
[38] Jiang NH,Zhu HL,Liu W,Fan C,Jin F,Xiang X. Metabolite differences of polyphenols in different litchi cultivars (Litchi chinensis Sonn.) based on extensive targeted metabonomics[J]. Molecules,2021,26 (4):1181. doi: 10.3390/molecules26041181
[39] 高晨曦,郭义红,孙威江,陈志丹. 基于DNA条形码和代谢组学技术的闽南乌龙茶产品鉴别[J]. 应用与环境生物学报,2021,27(6):1645−1652. Gao CX,Guo YH,Sun WJ,Chen ZD. Identification of oolong tea in southern Fujian,based on DNA barcoding and metabonomics[J]. Chinese Journal of Applied and Environmental Biology,2021,27 (6):1645−1652.
[40] Zhou BX,Wang ZH,Yin P,Ma BS,Ma CQ,et al. Impact of prolonged withering on phenolic compounds and antioxidant capability in white tea using LC-MS-based metabolomics and HPLC analysis:comparison with green tea[J]. Food Chem,2022,368:130855. doi: 10.1016/j.foodchem.2021.130855
[41] Liang LJ,Li WB,Tian M,Pan JJ,Feng ZY. Metabolomic profiling of five hulless barley (Hordeum vulgare L. ) with different:study on the metabolite difference of five hulless barley with different colors based on metabonomics approach[J]. Genet Resour Crop Evol,2022,69 (5):1843−1853. doi: 10.1007/s10722-022-01345-2
[42] 钱广涛,李祥羽,周静雯,张蘅,李立新. 三种不同颜色藜麦籽粒中氨基酸及其衍生物的代谢组学分析[J]. 植物生理学报,2021,57(11):2192−2202. Qian GT,Li XY,Zhou JW,Zhang H,Li LX. Metabonomic analysis of amino acids and derivatives in three types of quinoa grains with different colors[J]. Plant Physiology Journal,2021,57 (11):2192−2202.
[43] 于润众,宗恩祥,张丽媛,张东杰. 基于GC-MS分析不同品种小米代谢产物及代谢途径[J]. 食品科学,2021,42(24):199−205. Yu RZ,Zong EX,Zhang LY,Zhang DJ. Analysis of metabolites and metabolic pathways in different varieties of foxtail millets (Setaria italica L.) based on Gas Chromatography-Mass spectrometry[J]. Food Science,2021,42 (24):199−205.
[44] 张丽媛,于英博,赵子莹,于润众,李志江,张东杰. 不同品种绿豆中代谢产物的分离鉴定及代谢机制分析[J]. 食品科学,2021,42(16):169−175. Zhang LY,Yu YB,Zhao ZY,Yu RZ,Li ZJ,Zhang DJ. Separation and identification of metabolites and metabolic mechanism in two cultivars of mung bean[J]. Food Science,2021,42 (16):169−175.
[45] Espichán F,Rojas R,Quispe F,Cabanac G,Marti G. Metabolomic characterization of 5 native Peruvian chili peppers (Capsicum spp. ) as a tool for species discrimination[J]. Food Chem,2022,386:132704. doi: 10.1016/j.foodchem.2022.132704
[46] Chen JY,Wang WT,Kong JQ,Yue YD,Dong YY,et al. Application of UHPLC-Q-TOF MS based untargeted metabolomics reveals variation and correlation amongst different tissues of Eucommia ulmoides Oliver[J]. Microchem J,2022,172:106919. doi: 10.1016/j.microc.2021.106919
[47] Li MX,Liu MY,Wang BY,Shi L. Metabonomics analysis of stem extracts from Dalbergia sissoo[J]. Molecules,2022,27 (6):1982. doi: 10.3390/molecules27061982
[48] 魏海燕. 喀斯特山区不同环境因子对兰科植物分布的影响研究 — 以花江大峡谷为例[J]. 农村经济与科技,2020,31(1):68−70. [49] 贲蓓倍,徐维红,邹德玉,牟美睿,杨仁杰,刘海学. 不同施肥条件下的小麦籽粒代谢组学研究[J]. 麦类作物学报,2021,41(2):212−219. Ben BB,Xu WH,Zou DY,Mou MR,Yang RJ,Liu HX. Study on metabonomics of wheat grain under different fertilization conditions[J]. Journal of Triticeae Crops,2021,41 (2):212−219.
[50] 宋吉玲,周祖法,闫静,陆娜,程俊文,等. 基于UPLC-MS/MS技术的代谢组学方法研究麸皮对杨树桑黄代谢的影响[J]. 菌物学报,2021,40(3):641−655. Song JL,Zhou ZF,Yan J,Lu N,Cheng JW,et al. Study of effects of bran on the metabolism of Sanghuangporus vaninii based on metabolomics of UPLC-MS/MS[J]. Mycosystema,2021,40 (3):641−655.
[51] Liu HJ,Gu HR,Ye C,Guo CW,Zhu YF,et al. Planting density affects Panax notoginseng growth and ginsenoside accumulation by balancing primary and secondary metabolism[J]. Front Plant Sci,2021,12:628294. doi: 10.3389/fpls.2021.628294
[52] 刘龙桀,吴可心,刁云飞,李中跃,穆立蔷. 基于GC-MS分析东北红豆杉野生种与栽培种的代谢差异[J]. 植物研究,2021,41(5):798−806. Liu LJ,Wu KX,Diao YF,Li ZY,Mu LQ. Metabolic differences between wild and cultivated species of Taxus cuspidata based on GC-MS[J]. Bulletin of Botanical Research,2021,41 (5):798−806.
[53] Huang HZ,Tan P,Li MQ,Tan QC,Gao JH,et al. Quality analysis combined with mass spectrometry imaging reveal the difference between wild and cultivated Phyllanthus emblica Linn.: from chemical composition to molecular mechanism[J]. Arabian J Chem,2022,15 (6):103790. doi: 10.1016/j.arabjc.2022.103790
[54] Wu X,Liu Y,Guo JQ,Wang JX,Li MZ,et al. Differentiating Pu-erh raw tea from different geographical origins by 1H-NMR and U-HPLC/Q-TOF-MS combined with chemometrics[J]. J Food Sci,2021,86 (3):779−791. doi: 10.1111/1750-3841.15624
[55] 张舒,王长远,冯玉超,盛亚男,富天昕,等. 气相色谱-质谱联用代谢组学技术分析不同产地稻米代谢物[J]. 食品科学,2021,42(8):206−213. Zhang S,Wang CY,Feng YC,Sheng YN,Fu TX,et al. Analysis of metabolites in rice produced in different regions by GC-MS-based metabonomics[J]. Food Science,2021,42 (8):206−213.
[56] Bi WW,Zhao GX,Zhou YT,Xia XY,Wang JS,et al. Metabonomics analysis of flavonoids in seeds and sprouts of two Chinese soybean cultivars[J]. Sci Rep,2022,12 (1):5541. doi: 10.1038/s41598-022-09408-1
[57] 李富恒,张晓雯,张永芳,于萍,张宏发. 不同发育阶段老山芹种子多组学联合分析[J]. 东北农业大学学报,2021,52(10):32−46. Li FH,Zhang XW,Zhang YF,Yu P,Zhang HF. Integration analysis on multiple omics of Heracleum moellendorffii Hance seeds at different developmental stages[J]. Journal of Northeast Agricultural University,2021,52 (10):32−46.
[58] Wu XY,Wang YL,Tang HR. Quantitative metabonomic analysis reveals the germination associated dynamic and systemic biochemical changes for Mung-Bean (Vigna radiata) seeds[J]. J Proteome Res,2020,19 (6):2457−2470. doi: 10.1021/acs.jproteome.0c00181
[59] 肖金玲,沈蒙,葛云飞,康子悦,王娟,等. 萌发绿豆中多酚类物质动态变化规律及其抗氧化活性的研究[J]. 中国粮油学报,2020,35(7):28−35. Xiao JL,Shen M,Ge YF,Kang ZY,Wang J,et al. Dynamic changes and antioxidant activity of polyphenols in germinated mung bean[J]. Journal of the Chinese Cereals and Oils Association,2020,35 (7):28−35.
[60] Li Y,Wang JH,Wang KT,Lyu S,Ren LY,et al. Comparison analysis of widely-targeted metabolomics revealed the variation of potential astringent ingredients and their dynamic accumulation in the seed coats of both Carya cathayensis and Carya illinoinensis[J]. Food Chem,2022,374:131688. doi: 10.1016/j.foodchem.2021.131688
[61] 潘媛,赵晓,陈大霞. 灰毡毛忍冬花不同发育阶段的转录组学与代谢组学研究[J]. 中国中药杂志,2021,46(11):2798−2805. Pan Y,Zhao X,Chen DX. Different development phase of transcription proteomics and metabolomics of flower of Lonicera macranthoides[J]. China Journal of Chinese Materia Medica,2021,46 (11):2798−2805.
[62] 吴晓婷,张泽超,王红霞,张志华. 不同发育时期核桃内果皮的差异代谢物分析[J]. 河北农业大学学报,2021,44(4):44−50. Wu XT,Zhang ZC,Wang HX,Zhang ZH. Analysis of different metabolites in the endocarp of walnut at different developmental stages[J]. Journal of Hebei Agricultural University,2021,44 (4):44−50.
[63] Lee MY,Seo HS,Singh D,Lee SJ,Lee CH. Unraveling dynamic metabolomes underlying different maturation stages of berries harvested from Panax ginseng[J]. J Ginseng Res,2020,44 (3):413−423. doi: 10.1016/j.jgr.2019.02.002
[64] Liu JG,Liu YQ,Jia M,Kang XD,Wang SM,et al. Association of enriched metabolites profile with the corresponding volatile characteristics induced by rice yellowing process[J]. Food Chem,2021,349:129173. doi: 10.1016/j.foodchem.2021.129173
[65] Pan Y,Zhao X,Wang Y,Tan J,Chen DX. Metabolomics integrated with transcriptomics reveals the distribution of iridoid and crocin metabolic flux in Gardenia jasminoides Ellis[J]. PLoS One,2021,16 (9):e0256802. doi: 10.1371/journal.pone.0256802
-
期刊类型引用(0)
其他类型引用(1)
-
其他相关附件
-
DOCX格式
1 王梦迪 附表 1 点击下载(27KB)
-



 下载:
下载: