Research progress on DOG1, a key regulator of seed dormancy and germination
-
摘要:
DOG1(Delay of germination 1)蛋白是种子休眠的一种主要调控因子,可与ABA协同作用来延迟种子萌发。核心脱落酸(ABA)信号转导途径和DOG1途径在蛋白磷酸酶2C(PP2C)中汇聚。DOG1调控种子休眠需要PP2C,并通过与ABA过敏感萌发(AHG1/AHG3)结合来增强ABA的信号转导。DOG1抑制AHG1的作用来增加ABA的敏感性和诱导种子休眠。近年来,DOG1对种子休眠与萌发的调控研究取得了显著进展。本文对该领域的研究成果进行了综述,主要包括:DOG1对种子成熟、休眠与萌发的作用,DOG1表达的转录调控机制以及DOG1对种子休眠与萌发的调控及作用机制,最后提出了在本领域需要进一步研究的科学问题。
Abstract:Delay of germination 1 (DOG1) is a master regulator of seed dormancy and cooperates with abscisic acid (ABA) to delay seed germination. The core ABA signaling pathway and DOG1 pathway converge in protein phosphatase 2C (PP2C). DOG1 requires PP2C to regulate seed dormancy and enhances ABA signaling by binding to ABA HYPERSENSITIVE GERMINATION 1/3 (AHG1/AHG3). DOG1 suppresses AHG1 activity to increase ABA sensitivity and induce seed dormancy. In recent years, significant progress has been made in research on the regulation of seed dormancy and germination by DOG1. In this review, we summarize research achievements in this field, including the role of DOG1 in seed maturation, dormancy, and germination, the transcriptional regulatory mechanisms of DOG1 expression, and the regulation and action mechanism of DOG1 on seed dormancy and germination. We also highlight scientific issues that need to be further investigated in this field.
-
Keywords:
- ABA signaling /
- DOG1 regulator /
- Seed dormancy /
- Molecular mechanism /
- Transcription factors
-
土壤重金属污染是各方关注的严重环境问题之一。根据2014年《全国土壤重金属污染状况调查公报》,我国约有2 000万公顷耕地受到了重金属污染,其中330万公顷的污染程度达到了中度或重度污染。江西是我国矿产资源大省,铜资源在全国具有重要地位,长期的矿业开采、选矿、冶炼等产生的“三废”不断排放到周围环境,造成周边农田土壤铜污染严重,对农产品安全和人类身体健康都造成了极大的威胁[1]。以江西德兴铜矿区为例,周边土壤存在不同程度的铜污染,在919份调查样品中,铜含量位于50~400 mg/kg的有171个,含量大于400 mg/kg有18个,土壤最高铜含量可达1825 mg/kg,远大于中国土壤环境质量标准的三级标准(400 mg/kg)[2, 3],铜污染土壤的治理逐渐成为一个亟待解决的问题。植物修复(Phytoremediation)是一种新兴的土壤重金属净化技术[4],具有成本低廉、修复面积广、操作方便、二次污染易控制,美化植被环境等优点[5]。目前,关于修复植物的筛选多集中在草本植物,但草本生物量小、生长速度慢且很难进行机械收获作业,不适宜开展大面积污染土壤的修复[6],且农作物和草类等所吸收的重金属大多存在进入食物链或二次污染的风险,因此在实际修复应用中受到限制。当前,寻找生物量大、适应性强、观赏价值高且耐重金属的植物十分具有现实意义[7,8]。木本植物对重金属具有较强的耐性和一定的固定、吸收、累积能力,且其发达的深根系可使土壤中重金属移动性降低,降低其迁移和生物毒性,改善土壤质量等,引起研究人员的广泛关注[9]。
樟树(Cinnamomum camphora (L.) Presl)是重要的木本经济树种,樟脑、樟油等提取物是医药、化工、香料、防腐、农药的重要原料。樟树具有根系发达、生长迅速、生物量大、适应性强等优点,对重金属污染环境有较强的适应能力,15种常见乔木中,樟树对重金属的综合富集系数最高,对土壤中铜、锌和镉的富集能力均较强[10],在铜为主要污染源的土壤植物修复中有着较大的应用潜力。因此,樟树具有土壤修复收益型植物潜力。项目组前期将全国范围内收集的9个种源68个家系的樟树实生苗种植在以铜污染为主的江西德兴铜矿周边的水龙山实验修复基地,经过3年的跟踪调查,有12个家系适应性表现良好,成活率和保存率均较高,说明部分樟树具有较好的耐铜性,但樟树对铜离子的生理生化响应及富集转运特性还不清楚。本项目以前期筛选出的耐铜樟树为实验材料,通过盆栽实验研究不同浓度铜处理对樟树生长和生理指标的影响及铜的富集转运特性,探明耐铜樟树抵御铜毒性的生理生化机制,以期为樟树用于铜污染土壤修复提供科学依据。
1. 材料与方法
1.1 实验材料
实验于2021年7月在江西省科学院生物资源研究所人工气候室进行。实验地位于南昌市(28°41'N,115°59'E),实验材料为前期筛选出的耐铜樟树材料。收集同一单株的种子,将种子去果肉并反复搓洗干净,室内沙藏,于第2年春天播种,筛选长势一致的樟树幼苗统一移栽至事先准备好的基质中(红壤土∶细沙∶泥炭=1∶1∶1),栽培盆大小为上口径20 cm,底径14 cm,高14 cm,每盆基质重2.5 kg。经过3个月的缓苗,选取生长较好、高度一致的幼苗,采用随机区组设计,进行铜胁迫处理。
1.2 实验方法
以CuSO4·5H2O 为铜来源,配制铜浓度为100 g/kg的母液,处理前1周各栽培盆开始控水,至处理时各盆中基质均为干燥状态,处理时将各处理浓度所需母液用蒸馏水稀释至550 mL,缓缓浇灌至栽培盆中(经实验前反复验证,550 mL可以保证栽培盆内基质完全浸湿,且托盘里几乎没有多余水分流出),处理浓度分别为:0、50、150、300、600、900、1200 mg/kg(以基质重计算纯铜含量),每个处理4个重复。采样时间为处理60 d,每株选取3~4片嫩叶(枝条顶部开始倒数第 3~4 片叶)为待测材料,以锡箔纸包好,迅速投入液氮罐中,带回实验室放入−80 ℃冰箱保存,用于生理指标的测定;另准确称取0.1 g新鲜叶片,剪碎,放入事先准备好的装有95%乙醇的离心管中,黑暗处理,并放在摇床上震荡处理3 d,至叶片完全透明为止,用于色素含量的测定;最后将各处理单株分地上地下保存,放烘箱105 ℃杀青30 min,再70 ℃烘干至恒重测定干物质量,每株分别准确称取0.2 g根、茎、叶的研磨粉,微波消解,采用ICP-MS测定不同器官中铜含量。
1.3 生理指标测定
生理指标检测均参考施海涛[11]的方法。氯化硝基四氮唑蓝(NBT)光还原法测超氧化物歧化酶(SOD)活性;愈创木酚氧化法测过氧化物酶(POD)活性;高锰酸钾滴定法测过氧化氢酶(CAT)活性;烟酰胺腺嘌呤二核苷酸磷酸还原反应法测谷胱甘肽还原酶(GR)活性;硫代巴比妥酸比色法测丙二醛(MDA)含量;茚三酮比色法间接测定游离脯氨酸(Pro))含量;蒽酮比色法测定可溶性糖含量;考马斯亮蓝法测定可溶性蛋白含量。硫酸钛反应法测定过氧化氢(H2O2)含量,2-硝基苯甲酸反应法测谷胱甘肽(GSH)含量。所用试剂盒均由南京建成生物工程研究所提供,具体操作步骤按照相关说明书进行。
1.4 数据处理
所有指标的测定均进行4次生物学重复,最后结果采用平均值±标准差表示,采用SPSS 25软件作图并进行单因素方差分析(One-way ANOVA)。
2. 结果与分析
2.1 铜胁迫对樟树生长与生物量积累的影响
如图1所示,在铜胁迫浓度小于600 mg/kg时,樟树都可以很好地存活,根系和叶片均没有明显变化。其中150 mg/kg铜处理时,樟树长势最好,茎秆粗壮,叶片颜色墨绿。铜胁迫浓度为900 mg/kg时,叶片没有明显变化,部分根系开始出现褐化,根系的数量开始减少,但处理2个月后有新根萌出,可见900 mg/kg处理时,樟树依然可以存活。当处理浓度为1 200 mg/kg时,部分叶片开始萎蔫,根系褐化严重,且根系数量减少明显,也没有新根长出。因此在1 200 mg/kg铜处理下,樟树生长抑制及损害明显。
生物量干质量的结果与生长观察一致(表1),150 mg/kg处理时可以促进樟树的生长,其地上和根系干质量均高于其他处理,900 mg/kg处理时,地上干质量没有降低,反而高于对照组,但差异不显著(P>0.05);根系干质量稍有降低,差异也不显著(P>0.05)。1 200 mg/kg处理时,樟树的地上和根系干质量均低于其他各处理,且与150 mg/kg处理时差异显著(P<0.05),与其他处理差异不显著(P>0.05)。
表 1 不同浓度铜处理下樟树地上和根系生物量(干质量)的测定Table 1. Determination of aboveground and root biomass (dry mass) of Cinnamomum camphora treated with different concentrations of copper处理
Treatments / mg/kg地上质量
Dry mass of shoot / g根质量
Dry mass of root / g0 7.98±2.87bc 3.81±1.18ab 50 7.85±2.54bc 3.59±0.67ab 150 9.59±3.08a 4.53±1.35a 300 9.18±1.57ab 3.62±0.53ab 600 7.61±1.14bc 3.49±0.50ab 900 8.33±0.91bc 3.11±0.60b 1200 6.82±1.44c 2.97±0.90b 注:数据均为平均值±标准差,小写字母表示不同处理间在P<0.05水平上差异显著(n=4)。下同。 Notes: All data in the table are average values±standard deviation (SD). Lowercase letters after data in the same row indicate significant differences between different treatments at P<0.05 level (n=4). Same below. 2.2 铜胁迫对樟树光合色素含量的影响
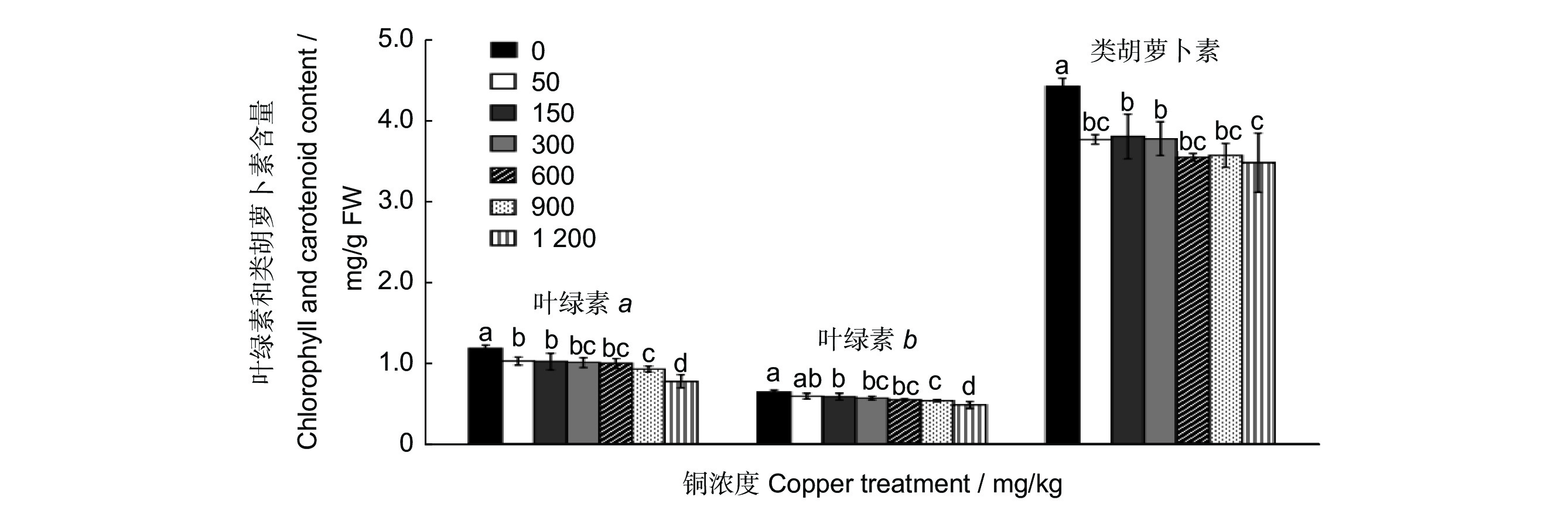
研究结果显示,叶绿素a、b及类胡萝卜素的含量均随着铜浓度的升高而降低。处理浓度为1200 mg/kg时,叶绿素a、b的含量均降至最低,显著低于其他各处理(P<0.05,图2);900 mg/kg处理时,叶绿素a、b的含量与300、600 mg/kg处理的差异均不显著(P>0.05),但与CK、50、150 mg/kg 处理时差异显著(P<0.05)。50、150、300、600 mg/kg 4种处理间的叶绿素a与叶绿素b差异均不显著(P>0.05)。类胡萝卜素含量的变化趋势与叶绿素a、b的变化趋势大致相同,也表现出随处理浓度的升高而降低的趋势,1 200 mg/kg处理时,类胡萝卜素含量最低,但与50、600、900 mg/kg 3个处理的差异不显著(P>0.05),与CK、150、300 mg/kg处理的差异显著(P<0.05)。
![]() 图 2 不同浓度铜处理60 d叶片中叶绿素a、b及类胡萝卜素含量的测定不同小写字母表示处理间在 P<0.05水平差异显著。下同。Figure 2. Determination of chlorophyll a, chlorophyll b, and carotenoid in Cinnamomum camphora leaves treated with different concentrations of copper for 60 dDifferent lowercase letters indicate significant differences between different treatments at P<0.05 level. Same below.
图 2 不同浓度铜处理60 d叶片中叶绿素a、b及类胡萝卜素含量的测定不同小写字母表示处理间在 P<0.05水平差异显著。下同。Figure 2. Determination of chlorophyll a, chlorophyll b, and carotenoid in Cinnamomum camphora leaves treated with different concentrations of copper for 60 dDifferent lowercase letters indicate significant differences between different treatments at P<0.05 level. Same below.2.3 铜胁迫对樟树抗氧化酶系统的影响
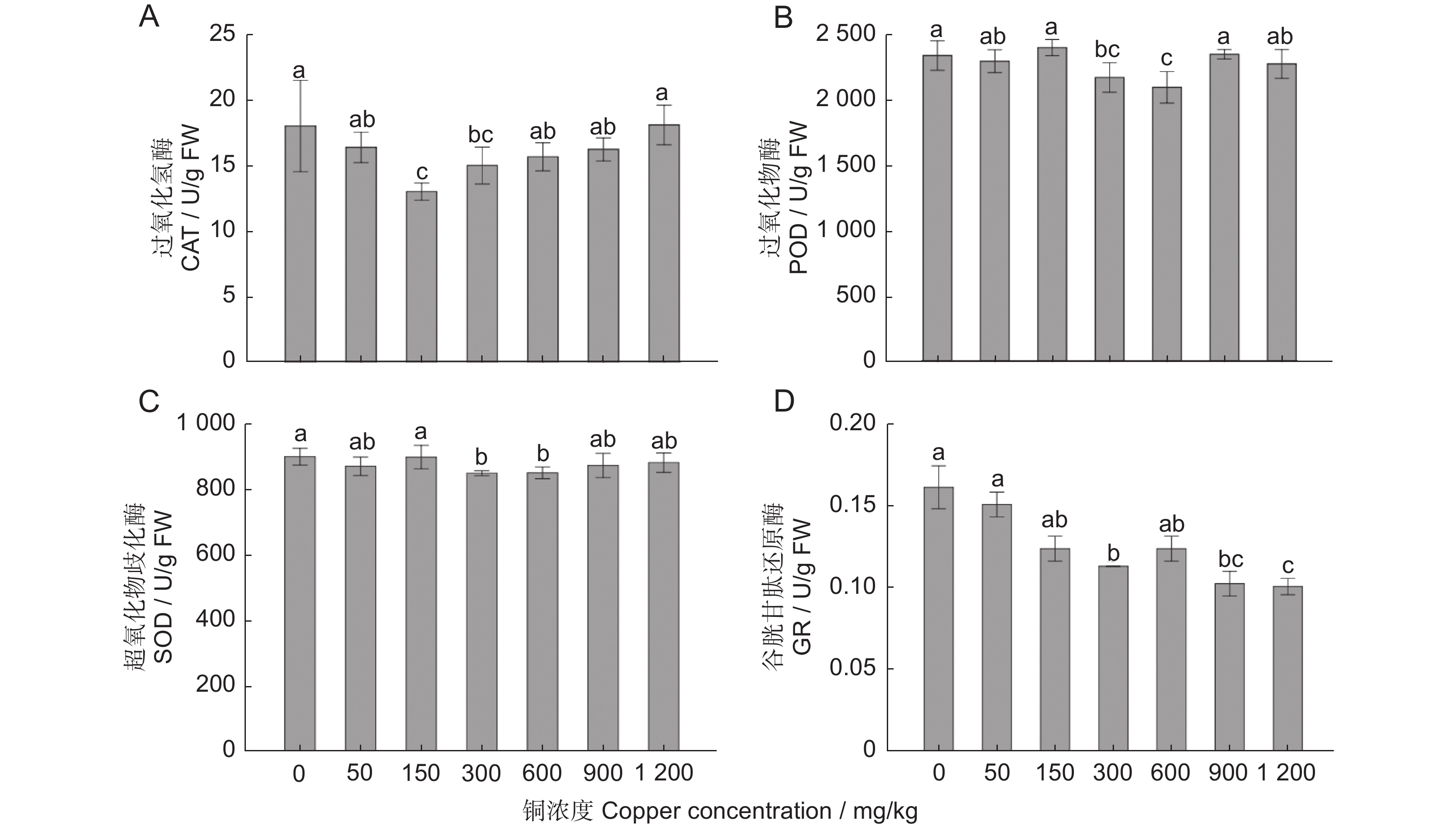
不同铜浓度影响樟树抗氧化酶系统的分析结果表明(图3),CAT活性在150 mg/kg时最低,与CK、50、600、900、1 200 mg/kg处理的差异显著(P<0.05;图3:A), CK和1 200 mg/kg两个处理时CAT活性最高,但与50、600、900 mg/kg处理间的差异不显著(P>0.05)。POD活性在CK、150、900 mg/kg处理时最高,显著高于300和600 mg/kg两个处理,600 mg/kg处理时的POD活性最低(图3:B)。SOD活性最高的是CK和150 mg/kg两个处理,显著高于300、600 mg/kg处理,与其他处理间的差异不显著(图3:C)。谷胱甘肽还原酶(GR)活性有随着处理浓度的升高逐渐降低的趋势,CK和50 mg/kg处理的GR活性最高,1 200 mg/kg处理时,活性最低,与CK、50、150、300、600 mg/kg处理差异显著,但与900 mg/kg处理的差异不显著(图3:D)
2.4 铜胁迫对樟树渗透调节物质含量及抗逆性指标的影响
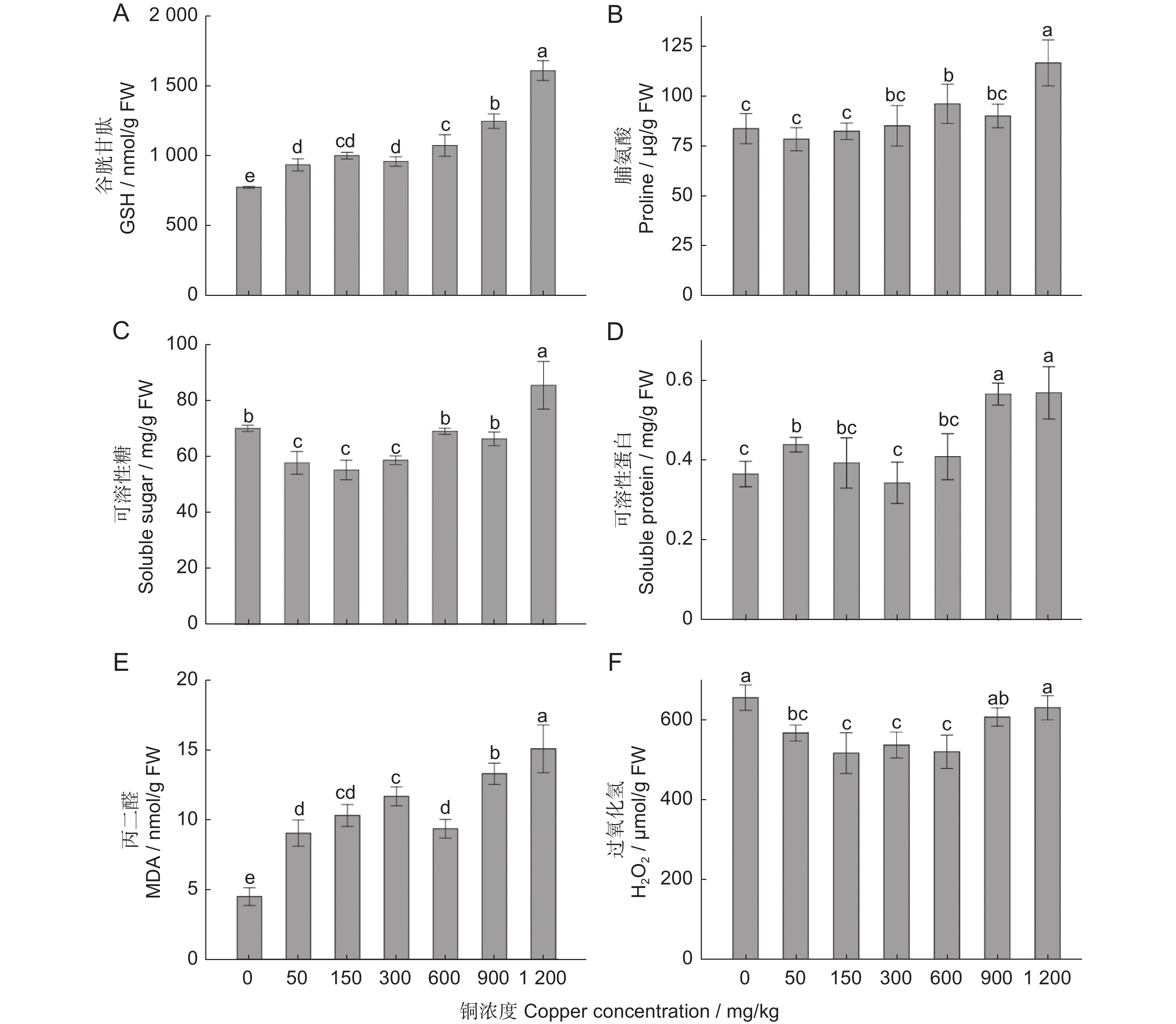
GSH作为植物体内重要的抗氧化剂和自由基清除剂,有随着处理浓度的升高而逐渐升高的趋势(图4:A)。渗透调节物质脯氨酸和可溶性糖含量均在1 200 mg/kg 处理时显著高于其他处理(P<0.05),其他处理间没有显著差异(P>0.05;图4:B、C)。可溶性蛋白在900和1 200 mg/kg 处理时显著高于其他处理,其他处理间差异不显著(图4:D)。随着铜浓度的升高,抗逆性指标MDA表现出逐渐升高的趋势(图4:E);H2O2浓度在CK和1 200 mg/kg两个处理时达到最高水平,显著高于50、150、300、600 mg/kg处理,与900 mg/kg处理的差异不显著(图4:F)。
2.5 铜胁迫对樟树不同组织铜浓度的影响
由表2可知,随着铜浓度的增加,根、茎、叶等不同组织的铜含量也逐渐升高,但与CK相比,处理浓度为50和150 mg/kg时,差异没有达到显著水平(P>0.05)。当处理浓度在300 mg/kg以上时,樟树叶、茎和根的铜含量均显著高于对照,但叶、茎的增幅显著低于根,与对照处理相比,300、600、900、1 200 mg/kg处理的叶片铜含量分别增加了1.73、1.80、1.74和2.12倍,茎部铜含量分别增加了1.42、1.45、1.35和2.02倍,而根部铜含量则分别增加了4.45、3.95、15.72和22.17倍。随着铜浓度的增加,铜离子由根系向叶片的转移率IF(Leaf/Root)由对照处理的0.078逐渐降至1 200 mg/kg处理时的0.007;铜离子由根系向茎的转移率IF(Stem/Root)由对照处理的0.06逐渐降至1 200 mg/kg处理时的0.005。樟树各器官铜含量的大小顺序为根>叶>茎,樟树主要将铜富集在根系,往地上茎和叶的转移率很低,且随着铜浓度的增大,往地上茎和叶的转移率也显著降低。
表 2 不同铜浓度处理下樟树不同部位铜含量及铜转移率的测定Table 2. Effects of different concentrations of copper on copper content and transfer rate in different parts of Cinnamomum camphora处理
Treatments /
mg/kg叶铜含量
Leaf Cu /
mg/kg茎铜含量
Stem Cu /
mg/kg根铜含量
Root Cu /
mg/kg铜转运
(叶/根)
IF (Leaf/Root)铜转运
(茎/根)
IF (Stem/Root)0 1.91±0.13c 1.46±0.29c 24.46±1.34d 0.078±0.005a 0.060±0.011a 50 1.72±0.01c 1.09±0.13c 25.20±1.28d 0.068±0.004b 0.044±0.007b 150 1.96±0.45c 1.40±0.23c 28.80±3.16d 0.067±0.009b 0.049±0.011ab 300 3.32±0.43b 2.08±0.24b 108.95±13.01c 0.031±0.002c 0.019±0.003c 600 3.44±0.37b 2.12±0.41b 96.67±11.71c 0.036±0.008c 0.022±0.006c 900 3.33±0.66b 1.97±0.36b 384.44±34.11b 0.009±0.002d 0.005±0.001d 1 200 4.06±0.53a 2.95±0.01a 542.27±51.01a 0.007±0.001d 0.005±0.001d 3. 讨论
3.1 铜胁迫对樟树生长的影响
樟树是重要的木本经济树种,茎叶是精油提取的主要器官,若能将樟树用于铜污染土壤修复中,不仅能改善生态环境,还可以实现收益,同时具有经济和生态效益。研究发现,樟树对铜污染有较好的适应能力[12, 13],在铜污染土壤的植物修复中应用潜力较大[14, 15]。本研究中,樟树对铜胁迫表现出“低促高抑”的变化趋势,一定浓度的铜(150 mg/kg)促进樟树的生长,其地上和根系生物量均高于其他处理,900 mg/kg处理时,樟树地上干质量没有降低,根系干重稍有降低,当处理浓度为1 200 mg/kg时,樟树的地上和根系干质量均降低。生长状况是反映植物对重金属耐受程度的直观指标[7],过量铜抑制植物生长曾多次被文献报道,例如,当Cu2+浓度高于20 μmol/L时,小白菜(Brassica campestris L. ssp. chinensis Makino)和芥菜(Brassica juncea L. Czern. et Coss.)的根、茎、叶生物量均随着铜浓度的升高而显著降低,铜胁迫抑制了小白菜和芥菜的生长[16, 17];玉米(Zea mays L.)幼苗的铜胁迫处理表明,Cu2+浓度为5 μmol/L时玉米幼苗的生长即受到抑制,随着胁迫浓度的升高,玉米幼苗地上和地下生物量均显著降低[18]。
光合作用是植物获取能量的重要途径,植物的能量含量与光合作用密切相关,叶绿素含量是反映植物耐受逆境胁迫的重要指标之一[19],本研究中叶绿素a、b及类胡萝卜素的含量均有随着铜处理浓度的升高而降低的趋势,与袁霞等[20]、李炎林等[21]的研究结果一致。当铜浓度为900 mg/kg时,叶绿素含量与其他低浓度处理差异均不显著;当铜浓度为1 200 mg/kg时,叶绿素含量则显著低于其他各处理。原因可能是重金属胁迫下,植物叶绿体膜结构受到一定程度的破坏,叶绿体开始降解,且叶绿体内的光合酶合成受抑制,叶片气孔关闭,光合作用强度降低[22],植物的生物量积累减少,生长受到抑制。
3.2 铜胁迫对樟树生理生化反应的影响
铜胁迫导致叶绿素含量降低及生长受抑制的主要原因是细胞内活性氧(ROS)含量不断增加并积累[23, 24]。低浓度的重金属可以激活植物的抗氧化应急系统,诱导 SOD、POD和CAT活性升高,以清除体内的活性氧[25],高浓度的 ROS则破坏细胞膜、核酸和叶绿素等关键成分,造成氧化损伤,影响植物的正常发育[26, 27]。MDA是细胞膜脂过氧化的产物,含量的高低反映了植物细胞膜脂过氧化的程度以及抗逆能力的强弱[25],MDA含量越高,植物细胞膜脂过氧化程度越高,ROS引起的细胞损伤越大[28]。本研究中MDA含量有随着铜处理浓度的升高而升高的趋势,900和1 200 mg/kg两个浓度处理时,MDA的含量显著高于其他各处理,说明高浓度铜胁迫对樟树细胞造成一定程度损伤。
可溶性蛋白、可溶性糖和游离脯氨酸作为植物体内重要的渗透调节物质,其含量的变化也是植物抗逆性强弱的重要反映。可溶性蛋白是植物细胞中含量最丰富的生物大分子之一,是植物结构和功能的重要物质基础,含量越高说明生理生化反应和代谢活动越强[29, 30]。本研究中,可溶性蛋白在铜浓度小于600 mg/kg时含量相对稳定,在900和1 200 mg/kg两个高浓度处理时,蛋白含量显著高于其他各处理,说明900和1 200 mg/kg两个浓度处理时,樟树的生理生化和代谢活动显著增强。游离脯氨酸既是渗透调节剂,又可作为自由基清除体内的重金属,其含量的增加可提高植物细胞液的浓度,维持细胞内外渗透压的平衡,保障细胞膜的结构稳定[31]。可溶性糖在维持细胞膜稳定、细胞扩张、酶活性和植株光合作用等方面起着重要作用[32],植物在逆境中可以通过增加可溶性糖等的含量来调节细胞的渗透压,提高对逆境的适应能力[33]。本研究中游离脯氨酸和可溶性糖的含量均在1 200 mg/kg处理时达到最高水平,显著高于其他各处理,900 mg/kg处理和其他低浓度处理的差异不显著,说明900 mg/kg处理时,细胞的内环境相对稳定。
植物抵抗重金属的能力与体内抗氧化酶系统的活性密切相关。POD、 CAT 与 SOD 协同作用,能够有效清除胁迫产生的活性氧,减少对植物细胞的危害,保证植物的正常生长[34]。过氧化氢是在铜胁迫下产生的活性氧物质,能直接或间接氧化细胞内的核酸、蛋白质等生物大分子,破坏细胞膜结构,从而加速细胞老化和解体,POD通过催化过氧化氢分解成无毒害的水和氧气,使植物体内的自由基保持在较低水平,从而避免对植物造成氧化伤害[35,36]。本研究中,不同铜处理后SOD、POD 和 CAT 酶的活性没有显著升高,过氧化氢的含量也相对稳定,在900 mg/kg处理时,其含量与对照差异不显著,说明该处理浓度没有超出樟树的自身调节能力,樟树可以有效抵抗铜的毒害作用。
3.3 铜胁迫对樟树不同器官铜含量的影响
植物对重金属的富集和转运能力是评价其耐受性的重要指标,与根系吸收及根系向地上部转运能力密切相关[9, 37]。本研究中,樟树主要将铜富集在根系,往地上茎和叶的转移率很低,且随着铜浓度的增加,往地上茎和叶的转移率逐渐降低,减轻了过量铜对樟树地上组织的毒害作用,与朱宇恩等[38]的研究结果一致。研究发现,植物主要通过两种途径抵抗铜的毒害作用:一是减少铜从根向地上部的转移,铜离子进入根系后,更多的集中在根系,从而降低铜对植物地上部的毒性;二是将铜离子集中在对铜毒性不太敏感的组织或部位固定起来,以减少铜在植物细胞中的转移,主要方式有将铜离子结合在细胞壁上或者转移到液泡中[39],或者利用植物体内的螯合剂与铜离子结合,形成对植物毒性较小或无毒的螯合物[40]。GSH为植物螯合剂(PCs)的合成提供合成前体,GR能够还原氧化型谷胱甘肽(GSSG)生成还原型谷胱甘肽(GSH),维持细胞内充足的GSH水平[41]。 本研究中,GSH含量有随着铜浓度的升高而升高的趋势,在900和1 200 mg/kg两个高浓度处理时,显著高于其他各处理;GR活性则在CK和50 mg/kg处理时活性最高,且随着处理浓度的升高逐渐降低,1 200 mg/kg处理时活性最低,推测GR被大量消耗,以合成更多的GSH参与植物螯合剂的合成并螯合过量铜离子。 但目前为止,樟树根系对铜离子的耐受策略及其分子调控机制还不清楚,深入探究樟树根系对铜离子的耐受和转运机制,是我们下一步要重点研究的内容。
-
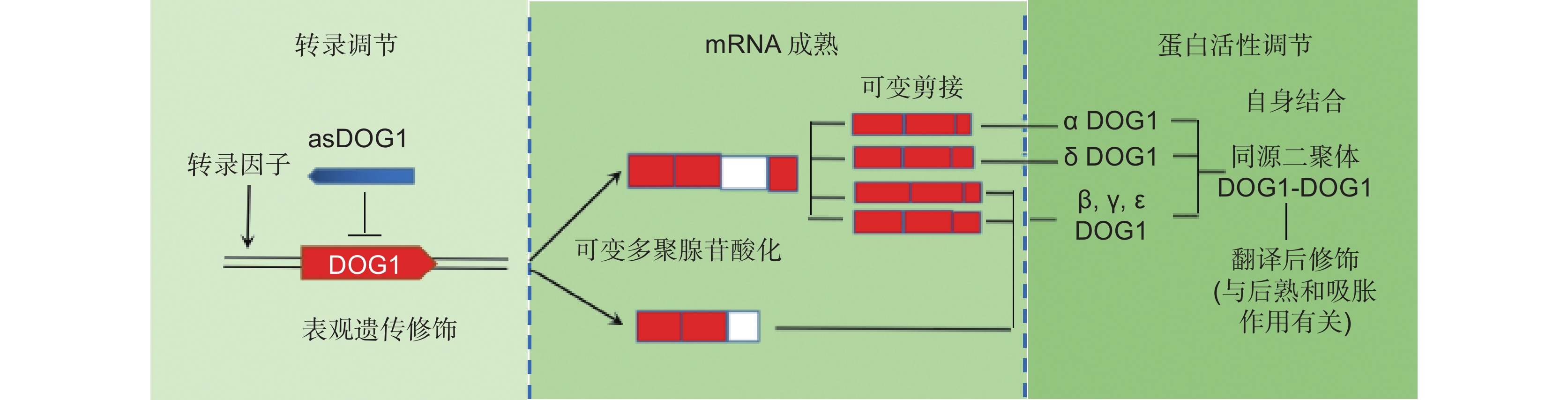
图 1 调控DOG1基因表达和蛋白活性的不同分子机制(改自Nonogaki[5]和Carrillo-Barral等[11]的文献)
DOG1的转录被表观遗传修饰和转录因子调控。此外,非编码反义序列(asDOG1)的转录作为DOG1表达的负调控因子起作用。由于拟南芥中存在两个多聚腺苷酸化位点,因此形成了两种不同的前体mRNA。前体mRNA通过可变剪接加工成为5种不同的成熟mRNA,随后翻译成为3种不同的蛋白异构体(β-、γ-和 ε-mRNA编码相同的蛋白异构体)。DOG1自身结合形成同源二聚体,能够发生与后熟和萌发过程有关的翻译后修饰。
Figure 1. Different molecular mechanisms regulating gene expression and protein activity of DOG1 (Redrawn from Nonogaki[5] and Carrillo-Barral et al.[11])
Transcription of DOG1 is regulated by epigenetic modifications and transcription factors. Additionally, transcription of the noncoding antisense sequence asDOG1 acts as a negative regulator of DOG1 expression. Two different precursor mRNAs are formed due to the existence of two polyadenylation sites in Arabidopsis thaliana. The precursor mRNAs are processed into five different mature mRNAs by alternative splicing and subsequently translated into three different protein isoforms (β-, γ-, and ε-mRNAs encode the same protein isoforms). DOG1 binds itself to form homodimers and undergoes post-translational modifications associated with after-ripening and germination processes.
图 2 DOG1和ABA信号转导在调控种子休眠/萌发中的作用机制(改自Nonogaki[13]的文献)
DOG1与AHG1(1种PP2C)结合,SnRK2被去抑制,ABA信号转导途径中的下游靶转录因子(例如碱性亮氨酸拉链蛋白、ABA不敏感5)被磷酸化,导致ABA的敏感性增加,种子处于休眠状态。相反,DOG1被转录后失活,AHG1与SnRK2物理结合,并使SnRK2失活,从而降低ABA的敏感性,种子萌发。DOG1通过提高ABA的敏感性增加种子的休眠。AHG1:ABA 过敏感萌发1;DOG1:萌发延迟1蛋白;PP2C: 蛋白磷酸酶2C;SnRK2:蔗糖非发酵-1-相关蛋白激酶2;TF:转录因子。
Figure 2. Mechanism of DOG1 and ABA signaling in regulating seed dormancy/germination (Redrawn from Nonogaki[13])
DOG1 binds to AHG1 (a PP2C), SnRK2 is de-repressed, and downstream target transcription factors (e.g., basic leucine zipper protein, ABA INSENSITIVE 5) in the ABA signaling pathway are phosphorylated, leading to increased ABA sensitivity and seed dormancy. In contrast, DOG1 is post-transcriptionally inactivated and AHG1 physically binds to and inactivates SnRK2, thereby reducing ABA sensitivity and seed germination. DOG1 enhances seed dormancy by increasing ABA sensitivity. AHG1: ABA hypersensitive germination 1; DOG1: Delay of germination 1; PP2C: Protein phosphatase 2C; SnRK2: Sucrose non-fermenting-1-related protein kinase 2; TF: Transcription factor.
图 3 DOG1和乙烯调控种子休眠/萌发的作用机制(改自Nonogaki[13]的文献)
ABA不敏感5-结合蛋白2(AFP2)是一种与NINJA相关的ABA不敏感5(ABI5)结合/抑制蛋白,与组蛋白脱乙酰酶(HDAC)和TPL(Topless)相互作用。AFP2与HDAC亚基和TPL辅阻遏物的相互作用表明,存在一种AFP2-TPL-HDAC复合物,该复合物可能抑制ABA信号转导和促进萌发。AHG1 PP2C是一种ABA信号转导的负调控因子,与AFP2和辅阻遏物相互作用。AHG1 PP2C使SnRK2和转录因子(TF,ABI5)失活,与AFP2和辅阻遏物TPL和FVE(IRA14多拷贝抑制因子)相互作用,表明其与抑制复合物相关,该抑制复合物通过使TF失活/转换和使TF靶子的染色质沉默来促进萌发。Ac:乙酰基;AHG1:ABA过敏感萌发1;APUM:拟南芥PUMILIO;ATXR7:拟南芥三胸相关7;bZIP67:碱性亮氨酸拉链蛋白67;CO:C重复;CTR1:组成性三重反应1;DOG1:萌发延迟1蛋白;DRE:脱水反应原件;E:乙烯;EIL:类EIN3;EIN:乙烯不敏感;ERF12:乙烯反应因子12;ETR1:乙烯受体1;GBL:类G盒基序;HUB1:H2B 单泛素化1;MET:甲基转移酶;NINJA:新型JAZ相互作用因子;P:磷酸;PAF1C:Pol Ⅱ相关因子1 复合物;Pol Ⅱ:RNA 聚合酶Ⅱ;PP2C:蛋白磷酸酶2C;RDO:休眠减少;SnRK2:蔗糖非发酵-1-相关蛋白激酶2;TFIIS:转录延长因子S-Ⅱ;TPR:Topless相关。
Figure 3. Action mechanism regulating seed dormancy/germination by DOG1 and ethylene (Redrawn from Nonogaki[13])
ABA insensitive 5-binding protein 2 (AFP2), a NINJA-related ABA insensitive 5 (ABI5)-binding/suppressing protein, interacts with histone deacetylase (HDAC) and TPL (Topless). AFP2 interaction with a HDAC subunit and TPL co-repressor suggests the presence of a AFP2-TPL-HDAC complex, which may suppress ABA signaling and promote germination. AHG1 PP2C, a negative regulator of ABA signaling, interacts with AFP2 and co-repressors. AHG1 PP2C, which inactivates SnRK2 and transcription factor (TF) ABI5, interacts with AFP2 and co-repressors TPL and FVE (multicopy suppressor of IRA14), suggesting association with the repressor complex, which promotes germination through TF inactivation/turnover and chromatin silencing of TF targets. Ac: Actyl group; AHG1: ABA hypersensitive germination 1; APUM: Arabidopsis thaliana PUMILIO; ATXR7: Arabidopsis thaliana trithorax-related 7; bZIP67: Basic leucine zipper protein 67; CO: C repeat; CTR1: Constitutive triple response 1; DOG1: Delay of germination 1; DRE: Dehydration responsive element; E: Ethylene; EIL: EIN3-like; EIN: Ethylene insensitive; ERF12: Ethylene response factor 12; ETR1: Ethylene receptor 1; GBL: G box-like motif; HUB1: H2B monoubiquitination 1; MET: Methyltransferase; NINJA: Novel interactor of JAZ; P: Phosphate; PAF1C: Pol Ⅱ-associated factor 1 complex; Pol Ⅱ: RNA polymerase Ⅱ; PP2C: Protein phosphatase 2C; RDO: Reduced dormancy; SnRK2: Sucrose non-fermenting-1-related protein kinase 2; TFIIS: Transcription elongation factor S-Ⅱ; TPR: Topless-related.
-
[1] Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. Seed: Physiology of Development, Germination and Dormancy[M]. 3rd ed. New York: Springer, 2013: 247-297.
[2] Donohue K,Dorn L,Griffith C,Kim ES,Aguilera A,et al. The evolutionary ecology of seed germination of Arabidopsis thaliana:variable natural selection on germination timing[J]. Evolution,2005,59(4):758−770.
[3] Ashikawa I,Mori M,Nakamura S,Abe F. A transgenic approach to controlling wheat seed dormancy level by using Triticeae DOG1-like genes[J]. Transgenic Res,2014,23(4):621−629. doi: 10.1007/s11248-014-9800-5
[4] Shu K,Liu XD,Xie Q,He ZH. Two faces of one seed:hormonal regulation of dormancy and germination[J]. Mol Plant,2016,9(1):34−45. doi: 10.1016/j.molp.2015.08.010
[5] Nonogaki H. Seed germination and dormancy:the classic story,new puzzles,and evolution[J]. J Integr Plant Biol,2019,61(5):541−563. doi: 10.1111/jipb.12762
[6] Iwasaki M,Penfield S,Lopez-Molina L. Parental and environmental control of seed dormancy in Arabidopsis thaliana[J]. Annu Rev Plant Biol,2022,73:355−378. doi: 10.1146/annurev-arplant-102820-090750
[7] Bentsink L,Jowett J,Hanhart CJ,Koornneef M. Cloning of DOG1,a quantitative trait locus controlling seed dormancy in Arabidopsis[J]. Proc Natl Acad Sci USA,2006,103(45):17042−17047. doi: 10.1073/pnas.0607877103
[8] Alonso-Blanco C,Bentsink L,Hanhart CJ,Vries HBD,Koornneef M. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana[J]. Genetics,2003,164(2):711−729. doi: 10.1093/genetics/164.2.711
[9] Nakabayashi K,Bartsch M,Xiang Y,Miatton E,Pellengahr S,et al. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION 1 protein levels in freshly harvested seeds[J]. Plant Cell,2012,24(7):2826−2838. doi: 10.1105/tpc.112.100214
[10] Li QJ,Chen X,Zhang SN,Shan SY,Xiang Y. DELAY OF GERMINATION 1,the master regulator of seed dormancy,integrates the regulatory network of phytohormones at the transcriptional level to control seed dormancy[J]. Curr Issues Mol Biol,2022,44(12):6205−6217. doi: 10.3390/cimb44120423
[11] Carrillo-Barral N,Rodríguez-Gacio MdC,Matilla AJ. Delay of Germination-1 (DOG1):a key to understanding seed dormancy[J]. Plants,2020,9(4):480. doi: 10.3390/plants9040480
[12] Nemati H,Sedghi M,Salekdeh GH,Afshari RT,Naghavi MR,et al. DELAY OF GERMINATION 1 (DOG1) regulates dormancy in dimorphic seeds of Xanthium strumarium[J]. Func Plant Biol,2022,49(8):742−758. doi: 10.1071/FP21315
[13] Nonogaki H. A repressor complex silencing ABA signaling in seeds?[J]. J Exp Bot,2020,71(10):2847−2853. doi: 10.1093/jxb/eraa062
[14] Carrillo-Barral N,Matilla AJ,García-Ramas C,del Carmen Rodríguez-Gacio M. ABA-stimulated SoDOG1 expression is after-ripening inhibited during early imbibition of germinating Sisymbrium officinale seeds[J]. Physiol Plant,2015,155(4):457−471. doi: 10.1111/ppl.12352
[15] Ashikawa A,Abe F,Nakamura S. DOG1-like genes in cereals:investigation of their function by means of ectopic expression in Arabidopsis[J]. Plant Sci,2013,208:1−9. doi: 10.1016/j.plantsci.2013.03.011
[16] Rikiishi K,Maekawa M. Seed maturation regulators are related to the control of seed dormancy in wheat (Triticum aestivum L.)[J]. PLoS One,2014,9(9):e107618. doi: 10.1371/journal.pone.0107618
[17] 张洋洋. 水稻DOG1-like基因克隆、表达分析与功能预测[D]. 北京: 中国科学院大学, 2016: 25-38. [18] Bewley JD, Nonogaki H. Seed maturation and germination[M]//Reference Module in Life Sciences. North York: Elsevier, 2017.
[19] Dekkers BJW,He HZ,Hanson J,Willems LAJ,Jamar DCL,et al. The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development[J]. Plant J,2016,85(4):451−465. doi: 10.1111/tpj.13118
[20] Zinsmeister J,Lalanne D,Terrasson E,Chatelain E,vandecasteele C,et al. ABI5 is a regulator of seed maturation and longevity in legumes[J]. Plant Cell,2016,28(11):2735−2754. doi: 10.1105/tpc.16.00470
[21] Leprince O,Pellizzaro A,Berriri S,Buitink J. Late seed maturation:drying without dying[J]. J Exp Bot,2017,68(4):827−841.
[22] Li P,Ni HH,Ying SB,Wei JL,Hu XY. Teaching an old dog a new trick:multifaceted strategies to control primary seed germination by DELAY OF GERMINATION 1 (DOG1)[J]. Phyton-Int J Exp Bot,2020,89(1):1−12.
[23] Chiang GCK,Bartsch M,Barua D,Nakabayashi K,Debieu M,et al. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana[J]. Mol Ecol,2011,20(16):3336−3349. doi: 10.1111/j.1365-294X.2011.05181.x
[24] Née G,Xiang Y,Soppe WJJ. The release of dormancy,a wake-up call for seeds to germinate[J]. Curr Opin Plant Biol,2017,35:8−14. doi: 10.1016/j.pbi.2016.09.002
[25] Yatusevich R,Fedak H,Ciesielski A,Krzyczmonik K,Kulik A,et al. Antisense transcription represses Arabidopsis seed dormancy QTL DOG1 to regulate drought tolerance[J]. EMBO Rep,2017,18(12):2186−2196. doi: 10.15252/embr.201744862
[26] Buijs G,Kodde J,Groot SPC,Bentsink L. Seed dormancy release accelerated by elevated partial pressure of oxygen is associated with DOG loci[J]. J Exp Bot,2018,69(15):3601−3608. doi: 10.1093/jxb/ery156
[27] Finch-Savage WE,Footitt S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments[J]. J Exp Bot,2017,68(4):843−856. doi: 10.1093/jxb/erw477
[28] Seo M,Marion-Poll A. Abscisic acid metabolism and transport[J]. Adv Bot Res,2019,92:1−49.
[29] Footitt S,Walley PG,Lynn JR,Hambidge AJ,Penfield S,et al. Trait analysis reveals DOG1 determines initial depth of seed dormancy,but not changes during dormancy cycling that result in seedling emergence timing[J]. New Phytol,2020,225(5):2035−2047. doi: 10.1111/nph.16081
[30] Murphey M,Kovach K,Elnacash T,He HZ,Bentsink L,et al. DOG1-imposed dormancy mediates germination responses to temperature cues[J]. Environ Exp Bot,2015,112:33−43. doi: 10.1016/j.envexpbot.2014.11.013
[31] Née G,Obeng-Hinneh E,Sarvari P,Nakabayashi K,Soppe WJJ. Secondary dormancy in Brassica napus is correlated with enhanced BnaDOG1 transcript levels[J]. Seed Sci Res,2015,25(2):221−229. doi: 10.1017/S0960258514000427
[32] Graeber K,Linkies A,Müller K,Wunchova A,Rott A,et al. Cross-species approaches to seed dormancy and germination:conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes[J]. Plant Mol Biol,2010,73(1-2):67−87. doi: 10.1007/s11103-009-9583-x
[33] Carbonero P,Iglesias-Fernández R,Vicente-Carbajosa J. The AFL subfamily of B3 transcription factors:evolution and function in angiosperm seeds[J]. J Exp Bot,2017,68(4):871−880.
[34] Pelletier JM,Kwong RW,Park S,Le BH,Baden R,et al. LEC1 sequentially regulates the transcription of genes involved in diverse developmental processes during seed development[J]. Proc Natl Acad Sci USA,2017,114(32):E6710−E6719.
[35] Dröge-Laser W,Snoek BL,Snel B,Weiste C. The Arabidopsis bZIP transcription factor family:an update[J]. Curr Opin Plant Biol,2018,45:36−49. doi: 10.1016/j.pbi.2018.05.001
[36] Bryant FN,Hughes D,Hassani-Pak K,Eastmond PJ. Basic LEUCINE ZIPPER TRANSCRIPTION FACTOR 67 transactivates DELAY OF GERMINATION 1 to establish primary seed dormancy in Arabidopsis[J]. Plant Cell,2019,31(6):1276−1288. doi: 10.1105/tpc.18.00892
[37] Yamamoto A,Kagaya Y,Usui H,Hobo T,Takeda S,et al. Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis[J]. Plant Cell Physiol,2010,51(12):2031−2046. doi: 10.1093/pcp/pcq162
[38] Sall K,Dekkers BJW,Nonogaki M,Katsuragawa Y,Koyari R,et al. DELAY OF GERMINATION 1-LIKE 4 acts as an inducer of seed reserve accumulation[J]. Plant J,2019,100(1):7−19. doi: 10.1111/tpj.14485
[39] Née G,Kramer K,Nakabayashi K,Yuan BJ,Xiang Y,et al. DELAY OF GERMINATION 1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy[J]. Nat Commun,2017,8(1):72. doi: 10.1038/s41467-017-00113-6
[40] Nishimura N,Tsuchiya W,Moresco JJ,Hayashi Y,Satoh K,et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme[J]. Nat Commun,2018,9(1):2132. doi: 10.1038/s41467-018-04437-9
[41] Molitor AM,Bu ZY,Yu Y,Shen WH. Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes[J]. PLoS Genet,2014,10(1):e1004091. doi: 10.1371/journal.pgen.1004091
[42] Fedak H,Palusinska M,Krzyczmonik K,Brzezniak L,Yatusevich R,et al. Control of seed dormancy in Arabidopsis by a cis-acting noncoding antisense transcript[J]. Proc Natl Acad Sci USA,2016,113(48):E7846−E7855.
[43] Kowalczyk J,Palusinska M,Wroblewska-Swiniarska A,Pietras Z,Szewc L,et al. Alternative polyadenylation of the sense transcript controls antisense transcription of DELAY OF GERMINATION 1 in Arabidopsis[J]. Mol Plant,2017,10(10):1349−1352. doi: 10.1016/j.molp.2017.07.011
[44] Liu YX,Koornneef M,Soppe WJJ. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy[J]. Plant Cell,2007,19(2):433−444. doi: 10.1105/tpc.106.049221
[45] Liu YX,Geyer R,van Zanten M,Carles A,Li Y,et al. Identification of the Arabidopsis REDUCED DORMANCY 2 gene uncovers a role for the polymerase associated factor 1 complex in seed dormancy[J]. PLoS One,2011,6(7):e22241. doi: 10.1371/journal.pone.0022241
[46] Mortensen SA,Sønderkær M,Lynggaard C,Grasser M,Nielsen KL,et al. Reduced expression of the DOG1 gene in Arabidopsis mutant seeds lacking the transcript elongation factor TFIIS[J]. FEBS Lett,2011,585(12):1929−1933. doi: 10.1016/j.febslet.2011.04.077
[47] Xiang Y,Nakabayashi K,Ding J,He F,Bentsink L,et al. Reduced dormancy5 encodes a protein phosphatase 2C that is required for seed dormancy in Arabidopsis[J]. Plant Cell,2014,26(11):4362−4375. doi: 10.1105/tpc.114.132811
[48] Mortensen SA,Grasser KD. The seed dormancy defect of Arabidopsis mutants lacking the transcript elongation factor TFIIS is caused by reduced expression of the DOG1 gene[J]. FEBS Lett,2014,588(1):47−51. doi: 10.1016/j.febslet.2013.10.047
[49] Zheng J,Chen FY,Wang Z,Cao H,Li XY,et al. A novel role for histone methyltransferase KYP/SUVH4 in the control of Arabidopsis primary seed dormancy[J]. New Phytol,2012,193(3):605−616. doi: 10.1111/j.1469-8137.2011.03969.x
[50] Nakabayashi K,Bartsch M,Ding J,Soppe WJJ. Seed dormancy in Arabidopsis requires self-binding ability of DOG1 protein and the presence of multiple isoforms generated by alternative splicing[J]. PLoS Genet,2015,11(12):e1005737. doi: 10.1371/journal.pgen.1005737
[51] Nonogaki H. Seed biology updates-highlights and new discoveries in seed dormancy and germination research[J]. Front Plant Sci,2017,8:524.
[52] Graeber K,Voegele A,Büttner-Mainik A,Sperber K,Mummenhoff K,et al. Spatiotemporal seed development analysis provides insight into primary dormancy induction and evolution of the Lepidium DELAY OF GERMINATION 1 genes[J]. Plant Physiol,2013,161(4):1903−1917. doi: 10.1104/pp.112.213298
[53] Szakonyi D,Duque P. Alternative splicing as a regulator of early plant development[J]. Front Plant Sci,2018,19:1174.
[54] Cyrek M,Fedak H,Ciesielski A,Guo YW,Sliwa A,et al. Seed dormancy in Arabidopsis is controlled by alternative polyadenylation of DOG1[J]. Plant Physiol,2016,170(2):947−955. doi: 10.1104/pp.15.01483
[55] Huo HQ,Wei SH,Bradford KJ. DELAY OF GERMINATION 1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways[J]. Proc Natl Acad Sci USA,2016,113(15):E2199−E2206.
[56] Bazin J,Langlade N,Vincourt P,Arribat S,Balzergue S,et al. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening[J]. Plant Cell,2011,23(6):2196−2208. doi: 10.1105/tpc.111.086694
[57] Huang DQ,Koh C,Feurtado JA,Tsang EWT,Cutler AJ. MicroRNAs and their putative targets in Brassica napus seed maturation[J]. BMC Genom,2013,14:140. doi: 10.1186/1471-2164-14-140
[58] Nonogaki H. ABA responses during seed development and germination[J]. Adv Bot Res,2019,92:171−217.
[59] 宋松泉,刘军,徐恒恒,刘旭,黄荟. 脱落酸代谢与信号传递及其调控种子休眠与萌发的分子机制[J]. 中国农业科学,2020,53(5):857−873. doi: 10.3864/j.issn.0578-1752.2020.05.001 Song SQ,Liu J,Xu HH,Liu X,Huang H. ABA metabolism and signaling and their molecular mechanism regulating seed dormancy and germination[J]. Sci Agr Sin,2020,53(5):857−873. doi: 10.3864/j.issn.0578-1752.2020.05.001
[60] Sano N,Marion-Poll A. ABA metabolism and homeostasis in seed dormancy and germination[J]. Int J Mol Sci,2021,22(10):5069. doi: 10.3390/ijms22105069
[61] Dejonghe W,Okamoto M,Cutler SR. Small molecule probes of ABA biosynthesis and signaling[J]. Plant Cell Physiol,2018,59(8):1490−1499. doi: 10.1093/pcp/pcy126
[62] Graeber K,Linkies A,Steinbrecher T,Mummenhoff K,Tarkowská D,et al. DELAY OF GERMINATION 1 mediates a conserved coat-dormancy mechanism for the temperature-and gibberellin-dependent control of seed germination[J]. Proc Natl Acad Sci USA,2014,111(34):E3571−E3580.
[63] Kendall SL,Hellwege A,Marriot P,Whalley C,Graham IA,et al. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors[J]. Plant Cell,2011,23(7):2568−2580. doi: 10.1105/tpc.111.087643
[64] Okamoto M,Kuwahara A,Seo M,Kushiro T,Asami T,et al. CYP707A1 and CYP707A2,which encode abscisic acid 8'-hydroxylases,are indispensable for proper control of seed dormancy and germination in Arabidopsis[J]. Plant Physiol,2006,141(1):97−107. doi: 10.1104/pp.106.079475
[65] Bentsink L,Hanson J,Hanhart CJ,Vries HBD,Coltrane C,et al. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways[J]. Proc Natl Acad Sci USA,2010,107(9):4264−4269. doi: 10.1073/pnas.1000410107
[66] Zhao ML,Yang SG,Liu XC,Wu KQ. Arabidopsis histone demethylases LDL1 and LDL2 control primary seed dormancy by regulating DELAY OF GERMINATION 1 and ABA signaling-related genes[J]. Front Plant Sci,2015,6:159.
[67] Kinoshita N,Berr A,Belin C,Chappuis R,Nishizawa NK,et al. Identification of growth insensitive to ABA3 (gia3),a recessive mutation affecting ABA signaling for the control of early post-germination growth in Arabidopsis thaliana[J]. Plant Cell Physiol,2010,51(2):239−251. doi: 10.1093/pcp/pcp183
[68] Teng S,Rognoni S,Bentsink L,Smeekens S. The Arabidopsis GSQ5/DOG1 Cvi allele is induced by the ABA-mediated sugar signalling pathway,and enhances sugar sensitivity by stimulating ABI4 expression[J]. Plant J,2008,55(3):372−381. doi: 10.1111/j.1365-313X.2008.03515.x
[69] Mönke G,Altschmied L,Tewes A,Reidt W,Mock HP,et al. Seed-specific transcription factors ABI3 and FUS3:molecular interaction with DNA[J]. Planta,2004,219(1):158−166. doi: 10.1007/s00425-004-1206-9
[70] Wang FF,Perry SE. Identification of direct targets of FUSCA3,a key regulator of Arabidopsis seed development[J]. Plant Physiol,2013,161(3):1251−1264. doi: 10.1104/pp.112.212282
[71] Lim J,Lim CW,Lee SC. Core components of abscisic acid signaling and their post-translational modification[J]. Front Plant Sci,2022,13:895698. doi: 10.3389/fpls.2022.895698
[72] Xue TT,Wang D,Zhang SZ,Ehlting J,Ni F,et al. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis[J]. BMC Genomics,2008,9:550. doi: 10.1186/1471-2164-9-550
[73] Li XY,Chen TT,Li Y,Wang Z,Cao H,et al. ETR1/RDO3 regulates seed dormancy by relieving the inhibitory effect of the ERF12-TPL complex on DELAY OF GERMINATION 1 expression[J]. Plant Cell,2019,31(4):832−884. doi: 10.1105/tpc.18.00449
-
期刊类型引用(1)
1. 龙桂根,黄芝云,吴南生,冯胜,冯超,丁菲,金松松,何利人,王勇,陈玲. 南酸枣种质资源果实性状变异和综合评价. 热带亚热带植物学报. 2024(06): 781-790 .  百度学术
百度学术
其他类型引用(3)




 下载:
下载: