Effects of salicylic acid on acteoside accumulation and gene expression in tuberous roots of Rehmannia glutinosa Libosch.
-
摘要:
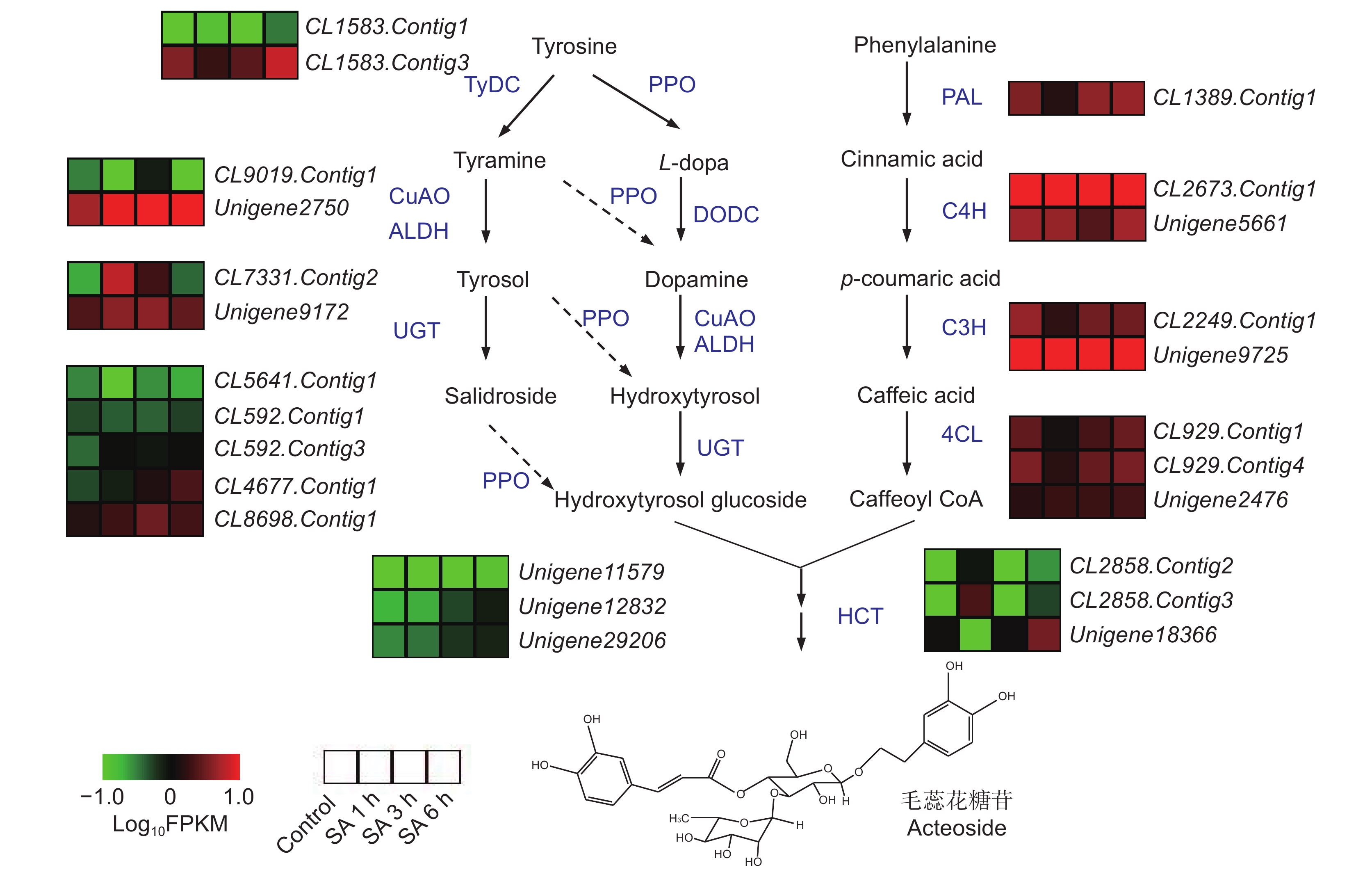
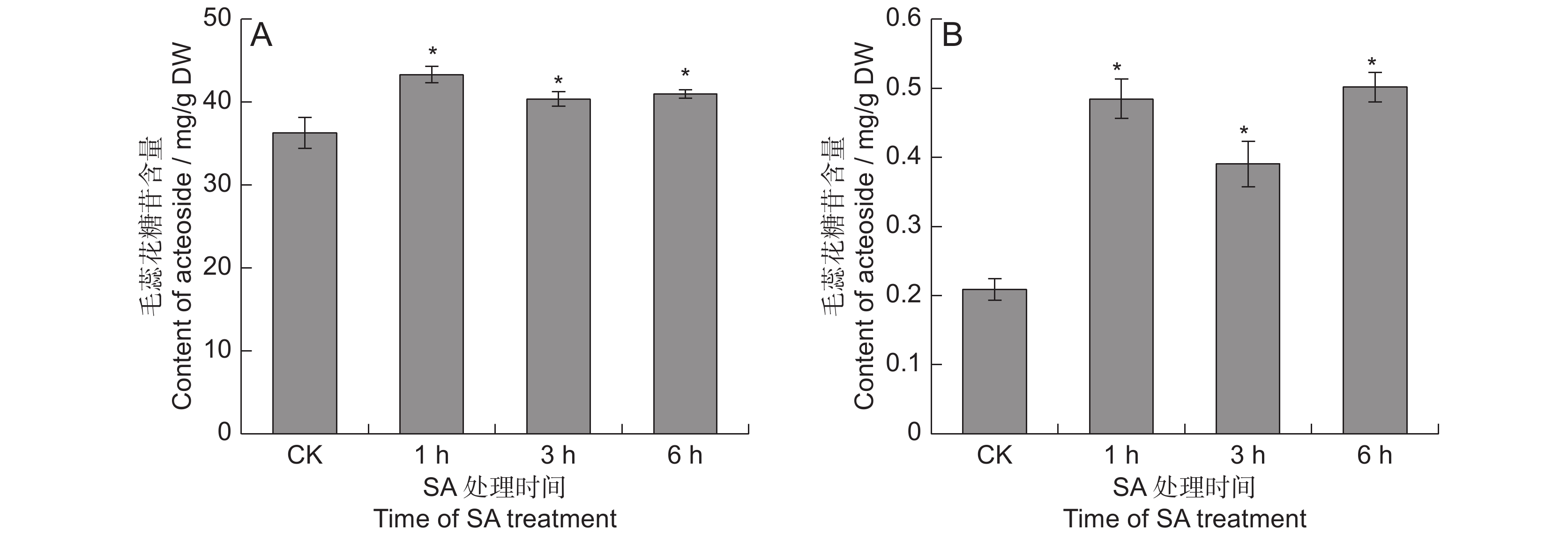
为了分析叶片表面喷施水杨酸(SA)对毛蕊花糖苷含量的影响及其分子调控特征,以地黄(Rehmannia glutinosa Libosch.)栽培品种‘温85-5’为材料,用100 μmol/L的SA喷施生长180 d的植株,在处理0、1、3和6 h后收集叶片和块根,测定毛蕊花糖苷的含量,并对块根进行转录组测序分析。结果显示,SA处理后,地黄叶片中的毛蕊花糖苷提高了11.2% ~ 19.3%,块根中的毛蕊花糖苷含量提高了0.9 ~ 1.4倍。转录组分析结果表明,SA处理3 h后的差异表达基因最多,且下调表达的基因多于上调表达。苯乙醇苷生物合成通路在差异表达基因中得到显著富集,毛蕊花糖苷合成途径的催化酶基因如ALDH、UGT和PPO等在SA处理后的地黄块根中上调表达。此外,本文还鉴定出多个在SA处理后差异表达的AP2-EREBP、WRKY和MYB等转录因子基因。
Abstract:The Rehmannia glutinosa ‘Wen 85-5’ cultivar was used to analyze the effects of spraying salicylic acid (SA) on the leaf surface on acteoside content and molecular regulation characteristics. The plants were grown for 180 days, then sprayed with SA (100 μmol/L). The leaves and tuberous roots of R. glutinosa were collected at 0, 1, 3, and 6 h after treatment to determine the content of acteoside. Transcriptome sequencing of the tuberous roots at different times after SA treatment was also performed. Results showed that, compared with the controls, acteoside content in the leaves and tuberous roots increased by 11.2% - 19.3%, and 0.9 - 1.4 times, respectively, after SA treatment. Transcriptome analysis showed that most differentially expressed genes (DEGs) were obtained 3 h after SA treatment, with more down-regulated genes than up-regulated genes. Most DEGs were significantly enriched in the phenylpropanoid biosynthesis pathway, while several catalytic enzyme genes of the acteoside synthesis pathway, such as ALDH, UGT, and PPO, were up-regulated in the tuberous roots. Many AP2-EREBP, WRKY, and MYB transcription factor genes were differentially expressed after SA treatment. This study provides theoretical support for the use of elicitors to treat R. glutinosa plants in the field to increase acteoside content.
-
Keywords:
- Rehmannia glutinosa /
- Salicylic acid /
- Foliar-spraying /
- Acteoside /
- Gene expression
-
-
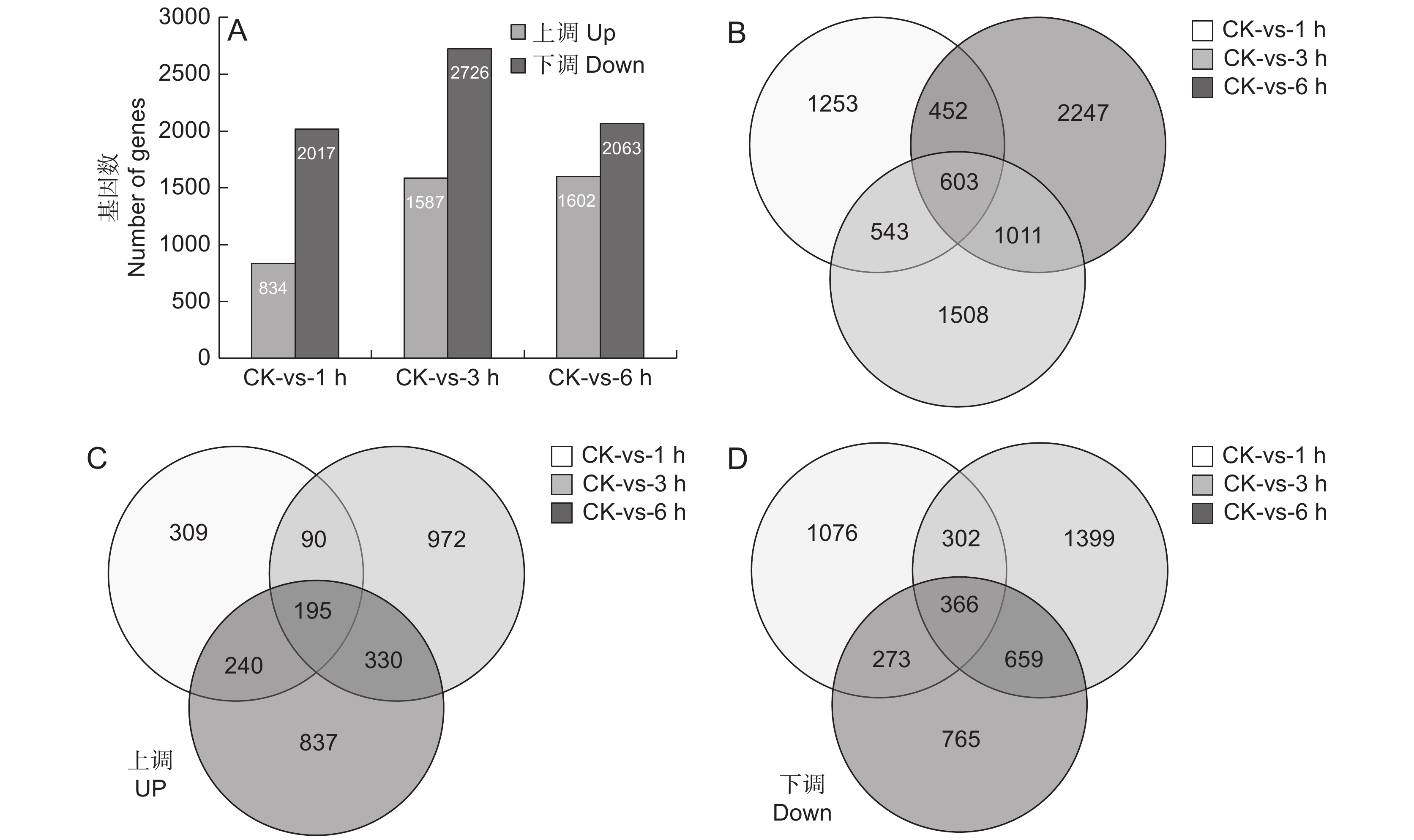
图 2 SA处理后基因显著差异表达
A:SA处理过程中上调和下调的基因数目; B ~ D:SA处理后不同时间点鉴别出的总DGEs(B)、上调DGEs(C)和下调DGEs的维恩图。
Figure 2. Significant DEGs in response to SA treatment
A: Up-regulated and down-regulated gene numbers during SA treatment; B − D: Venn diagram of total DEGs (B), up-regulated DEGs (C), and down-regulated DEGs (D) identified at different time points after SA treatment.
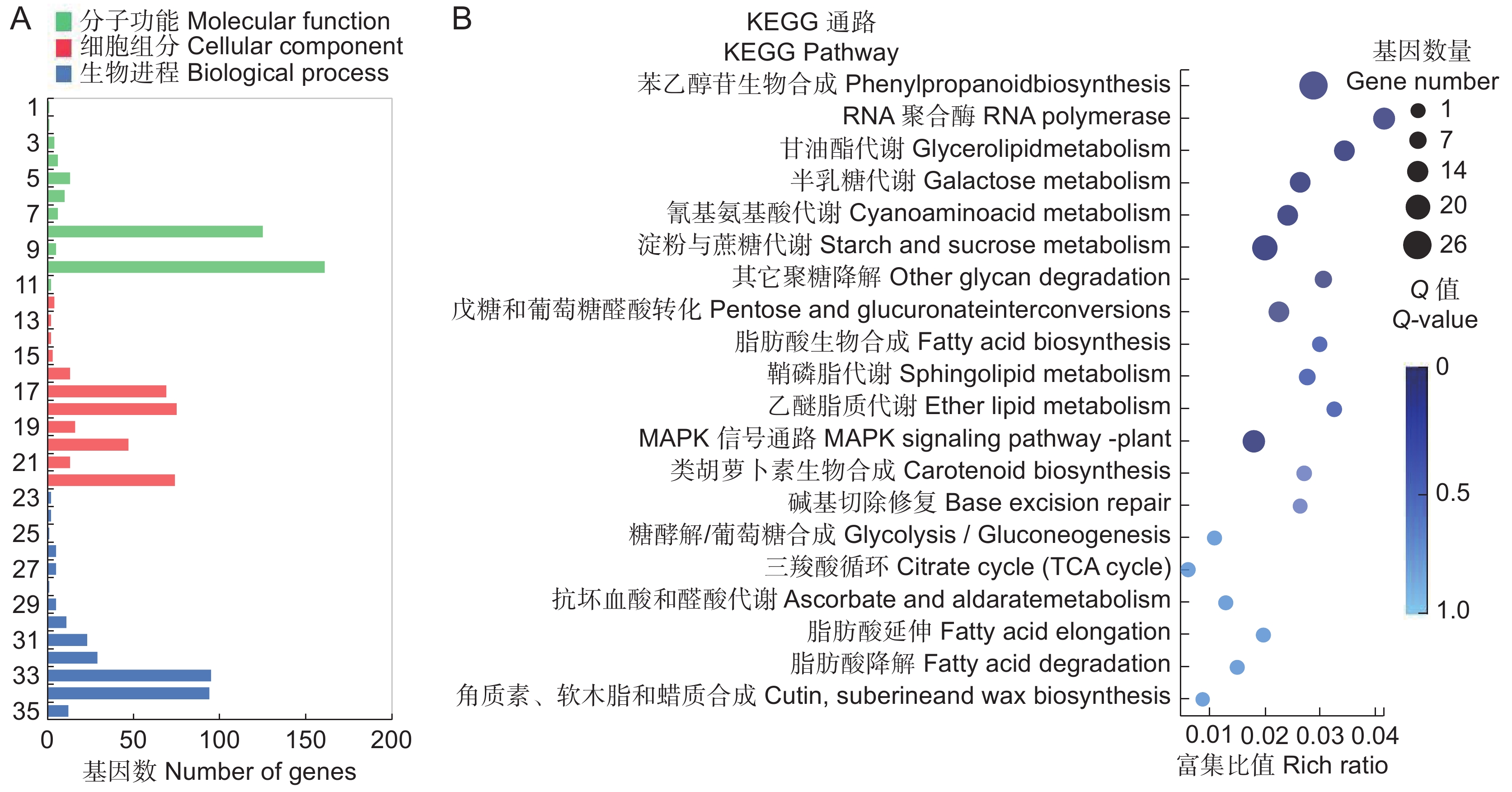
图 3 SA处理后3种比对均差异表达基因的GO分类及KEGG通路富集
A:603个基因的GO分类;B:KEGG富集的前20个代谢通路。圆点大小和颜色分别表示通路中DEGs的数量和Q值范围。1:分子转导活性;2:分子载体活性;3:信号转导活性;4:结构分子活性;5:转运活性;6:转录调节活性;7:抗氧化活性;8:结合;9:分子功能调节;10:催化活性;11:膜封闭腔;12:超分子复合物;13:共质体;14:细胞连接;15:细胞组分;16:细胞外区域;17:膜组分;18:膜;19:细胞器组分;20:细胞器;21:大分子复合物;22:细胞;23:生殖过程;24:繁殖;25:解毒作用;26:多细胞生物过程;27:发育过程;28:多生物体过程;29:信号;30:定域化;31:生物调节;32:对刺激的反应;33:代谢过程;34:细胞过程;35:细胞成分组织或生物合成。
Figure 3. GO classification and KEGG pathway enrichment of co-DEGs in three comparisons
A: GO classification of 603 genes; B: Top 20 enriched KEGG pathways among 603 genes. Size and color of dot represent number and scope of DEGs in pathway, respectively. 1: Molecular transducer activity; 2: Molecular carrier activity; 3: Signal transducer activity; 4: Structural molecule activity; 5: Transporter activity; 6: Transcription regulator activity; 7: Antioxidant activity; 8: Binding; 9: Molecular function regulator; 10: Catalytic activity; 11: Membrane-enclosed lumen; 12: Supramolecular complex; 13: Symplast; 14: Cell junction; 15: Cell part; 16: Extracellular region; 17: Membrane part; 18: Membrane; 19: Organelle part; 20: Organelle; 21: Macromolecular complex; 22: Cell; 23: Reproductive process; 24: Reproduction; 25: Detoxification; 26: Multicellular organismal process; 27: Developmental process; 28: Multi-organism process; 29: Signaling; 30: Localization; 31: Biological regulation; 32: Response to stimulus; 33: Metabolic process; 34: Cellular process; 35: Cellular component organization or biogenesis.
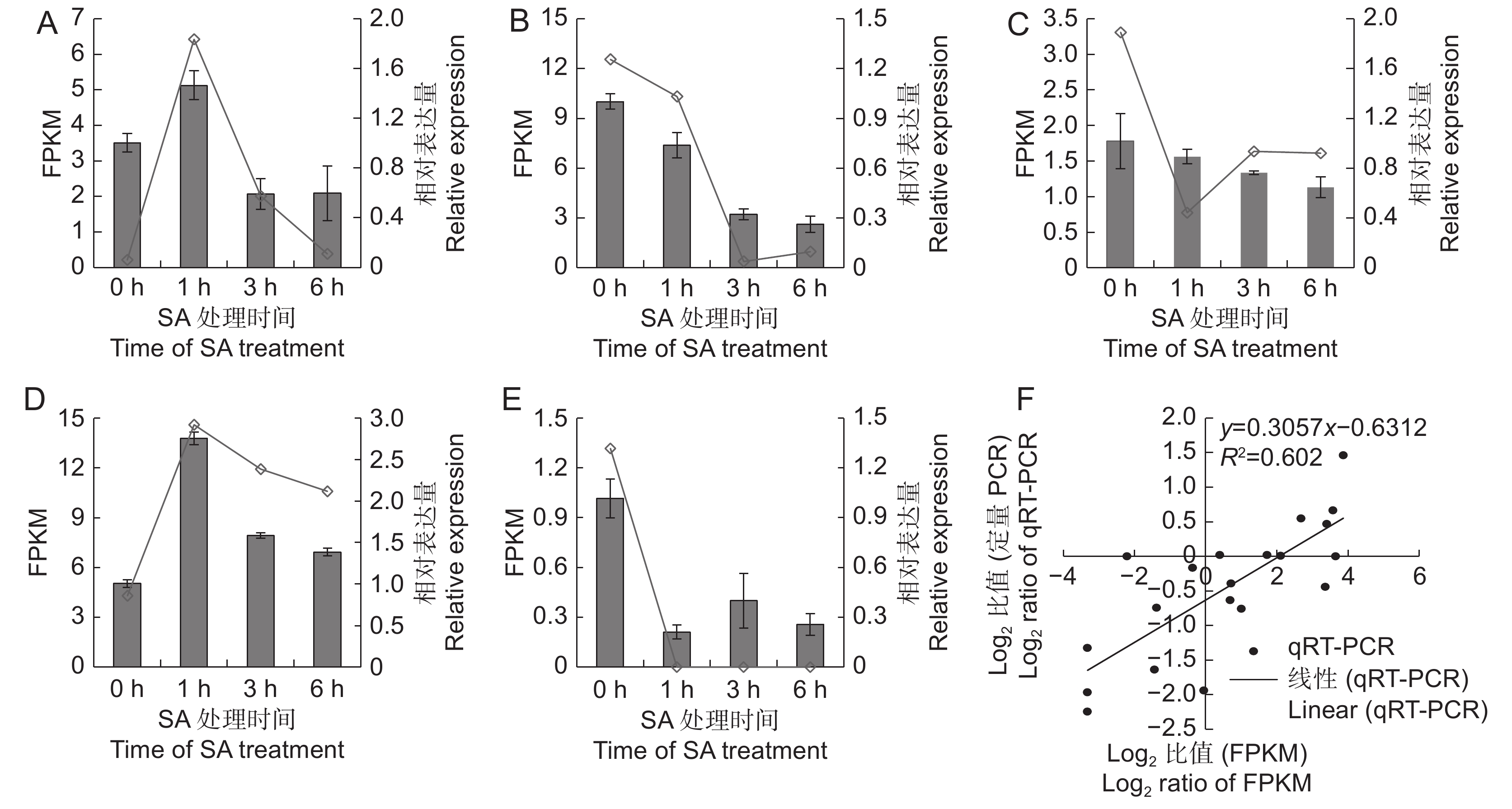
图 5 差异表达基因的qRT-PCR验证
A ~ E分别为CL7331.Contig2、Unigene1886、CL985.Contig1、CL5931.Contig2和CL379.Contig3的FPKM值与相对表达量;F:FPKM值与qRT-PCR的相关性分析。
Figure 5. Validation of DEGs by qRT-PCR
A–E: Represent expression and FPKM values of CL7331.Contig2, Unigene1886, CL985.Contig1, CL5931.Contig2, and CL379.Contig3; F: Correlation analysis between FPKM and qRT-PCR data.
表 1 测序数据统计结果
Table 1 Statistics of sequenced data
样本
Sample总原始序列
Total raw reads / M总测序序列
Total clean reads / M总测序碱基数
Total clean bases / Gb测序序列比率
Clean read ratio / %总匹配率
Total mapped / %特异匹配率
Uniquely mapped / %Control_1 21.94 21.13 1.06 96.3 87.70 51.31 Control_2 21.94 21.07 1.05 96.04 86.92 51.18 Control_3 21.94 21.08 1.05 96.08 85.72 51.09 SA 1h_1 21.94 21.27 1.06 96.93 85.44 47.35 SA 1h_2 21.94 21.08 1.05 96.05 87.07 48.03 SA 1h_3 21.94 21.15 1.06 96.37 87.28 49.26 SA 3h_1 21.94 20.98 1.05 95.59 86.85 50.36 SA 3h_2 21.94 21.05 1.05 95.93 85.74 49.92 SA 3h_3 21.94 21.05 1.05 95.95 86.81 50.47 SA 6h_1 21.94 21.09 1.05 96.11 86.50 50.25 SA 6h_2 21.94 21.06 1.05 96.00 85.04 49.42 SA6h_3 21.94 21.03 1.05 95.86 86.68 49.97 表 2 SA处理后差异表达的转录因子数
Table 2 Number of differentially expressed transcription factors (TFs) after SA treatment
转录因子
Transcription factorCK-vs-1 h CK-vs-3 h CK-vs-6 h 共同差异表达的基因数
Number of common DEGs下调 Down 上调 Up 下调 Down 上调 Up 下调 Down 上调 Up zf-HD 2 1 1 1 0 0 0 WRKY 11 5 8 16 5 15 5 TUB 1 0 1 1 0 0 0 Trihelix 1 0 1 0 0 0 0 Tify 1 0 3 0 3 0 1 PLATZ 0 4 0 4 1 3 2 SRS 0 0 2 0 2 0 0 OFP 2 0 3 0 1 0 0 NAC 2 8 2 6 0 8 4 MYB 15 10 5 7 6 12 3 mTERF 1 0 2 0 1 1 0 MADS 2 2 3 4 2 2 1 LOB 1 0 4 2 2 0 0 HSF 5 2 4 6 3 6 2 GRAS 1 8 0 4 0 7 2 G2-like 2 0 2 1 0 3 0 CPP 2 0 2 0 4 0 2 C2H2 3 1 8 1 3 1 2 C2C2-GATA 2 0 1 0 5 0 0 C2C2-Dof 1 1 3 0 2 1 0 C2C2-CO-like 0 1 0 2 4 3 1 bZIP 1 2 0 0 1 0 0 bHLH 12 3 15 4 5 4 2 AP2-EREBP 15 12 12 18 9 21 6 ABI3VP1 5 2 7 1 1 1 0 总数 88 62 89 78 60 88 33 表 3 SA处理后上调表达的转录因子基因
Table 3 Up-regulated transcription factor genes after SA treatment
转录因子
Transcription factor基因
GeneLog2(SA/CK) 功能
Function1 h 3 h 6 h AP2-EREBP CL1637.Contig3 2.82 1.29 2.42 Ethylene-responsive transcription factor ERF071 AP2-EREBP CL4501.Contig2 1.66 1.75 2.16 Pathogenesis-related genes transcriptional activator PTI6 AP2-EREBP CL7827.Contig1 1.77 3.51 4.24 Ethylene-responsive transcription factor ERF106-like AP2-EREBP Unigene2558 1.62 1.06 1.74 Ethylene-responsive transcription factor 2-like MYB CL1983.Contig1 4.55 4.19 3.12 Transcription factor TFIIIB component B''-like MYB CL4303.Contig1 3.76 2.61 3.47 Single MYB histone protein NAC CL2945.Contig2 6.82 7.15 7.51 NAC domain-containing protein 82-like isoform X1 NAC CL4851.Contig1 3.15 1.99 2.44 NAC transcription factor 29 NAC CL4851.Contig2 3.15 2.27 2.82 NAC transcription factor 29 NAC Unigene5193 2.09 1.38 2.37 NAC domain-containing protein 72 C2C2-CO-like CL5505.Contig3 2.94 2.66 4.31 Zinc finger protein CONSTANS-LIKE 4-like PLATZ CL5569.Contig1 2.31 1.49 2.53 Interleukin-1 receptor-associated kinase 4 PLATZ CL5569.Contig3 2.47 1.71 2.38 Interleukin-1 receptor-associated kinase 4 GRAS CL645.Contig2 1.93 1.65 2.91 Scarecrow-like protein 14 GRAS Unigene10453 1.03 1.03 1.08 Scarecrow-like protein 15 WRKY CL6521.Contig1 2.84 1.66 2.35 Probable WRKY transcription factor 25 WRKY CL7324.Contig3 1.13 1.59 1.21 Probable WRKY transcription factor 35 WRKY CL791.Contig6 2.59 2.28 2.29 Probable WRKY transcription factor 40 bHLH Unigene12420 2.20 2.20 2.29 Phytochrome-interacting factor 3 MADS Unigene23315 2.17 3.01 2.27 MADS-box transcription factor -
[1] 解杨,钟凌云,王卓,宋金菊,李家晴,等. 地黄炮制历史沿革及现代研究进展[J]. 中国实验方剂学杂志,2022,28(2):273−282. Xie Y,Zhong LY,Wang Z,Song JJ,Li JQ,et al. Historical evolution and modern research progress of Rehmanniae radix[J]. Chinese Journal of Experimental Traditional Medical Formulae,2022,28 (2):273−282.
[2] 国家药典委员会. 中华人民共和国药典: 一部[M]. 北京: 中国医药科技出版社, 2020: 129−130. [3] 陈金鹏,张克霞,刘毅,盖晓红,任涛,等. 地黄化学成分和药理作用的研究进展[J]. 中草药,2021,52(6):1772−1784. doi: 10.7501/j.issn.0253-2670.2021.06.028 Chen JP,Zhang KX,Liu Y,Gai XH,Ren T,et al. Research progress on chemical constituents and pharmacological actions of Rehmannia glutinosa[J]. Chinese Traditional and Herbal Drugs,2021,52 (6):1772−1784. doi: 10.7501/j.issn.0253-2670.2021.06.028
[4] 王丰青,杨旭,左鑫,苗春妍,张重义. 地黄全长转录组测序及苯乙醇苷合成途径催化酶基因鉴定[J]. 药学学报,2022,57(3):831−838. Wang FQ,Yang X,Zuo X,Miao CY,Zhang ZY. Full-length transcriptome sequence and identification of genes involved in phenylethanol glycoside biosynthesis in Rehmannia glutinosa[J]. Acta Pharmaceutica Sinica,2022,57 (3):831−838.
[5] 王丰青,王丽娜,智惊宇,张苗,杨超飞,等. 不同品种地黄中毛蕊花糖苷的动态积累规律变化[J]. 中国实验方剂学杂志,2017,23(24):78−83. doi: 10.13422/j.cnki.syfjx.2017240078 Wang FQ,Wang LN,Zhi JY,Zhang M,Yang CF,et al. Changes in dynamic accumulation of acteoside from different Rehmannia glutinosa cultivars[J]. Chinese Journal of Experimental Traditional Medical Formulae,2017,23 (24):78−83. doi: 10.13422/j.cnki.syfjx.2017240078
[6] 柴茂,董诚明,江道会,姚峰,王秀书. 不同品种怀地黄中梓醇和毛蕊花糖苷的高效液相色谱法测定[J]. 中医学报,2013,28(5):690−692. doi: 10.16368/j.issn.1674-8999.2013.05.050 Chai M,Dong CM,Jiang DH,Yao F,Wang XS. Determination of catalpol and verbascoside of different cultivars Rehmannia glutinosa Libosch. by high performance liquid chromatography[J]. China Journal of Chinese Medicine,2013,28 (5):690−692. doi: 10.16368/j.issn.1674-8999.2013.05.050
[7] 柴烨,郑立颖. 不同产地生地黄和熟地黄中毛蕊花糖苷含量的比较研究[J]. 甘肃科学学报,2015,27(3):37−40. doi: 10.16468/j.cnki.issn1004-0366.2015.03.010 Chai Y,Zheng LY. A comparative study of verbascoside content in Rehmannia root & prepared Rehmannia root from different places of production[J]. Journal of Gansu Sciences,2015,27 (3):37−40. doi: 10.16468/j.cnki.issn1004-0366.2015.03.010
[8] 王丰青,杨超飞,李铭铭,左鑫,杨旭,等. 密度对地黄生长及基因转录特性的影响分析[J]. 中国中药杂志,2021,46(17):4367−4379. doi: 10.19540/j.cnki.cjcmm.20210623.101 Wang FQ,Yang CF,Li MM,Zuo X,Yang X,et al. Effects of density on growth and gene transcription characteristics of Rehmannia glutinosa[J]. China Journal of Chinese Materia Medica,2021,46 (17):4367−4379. doi: 10.19540/j.cnki.cjcmm.20210623.101
[9] 王丰青,李欣容,杨超飞,智惊宇,张雪丽,等. 遮阴对地黄块根性状、光合特性及基因转录的影响[J]. 中草药,2019,50(18):4419−4429. doi: 10.7501/j.issn.0253-2670.2019.18.023 Wang FQ,Li XR,Yang CF,Zhi JY,Zhang XL,et al. Effects of shading on tuberous root traits,photosynthetic characteristics and gene transcription of Rehmannia glutinosa[J]. Chinese Traditional and Herbal Drugs,2019,50 (18):4419−4429. doi: 10.7501/j.issn.0253-2670.2019.18.023
[10] Wang FQ,Zhi JY,Zhang ZY,Wang LN,Suo YF,et al. Transcriptome analysis of salicylic acid treatment in Rehmannia glutinosa hairy roots using RNA-seq technique for identification of genes involved in acteoside biosynthesis[J]. Front Plant Sci,2017,8:787. doi: 10.3389/fpls.2017.00787
[11] Li MJ,Yang YH,Feng FJ,Zhang B,Chen SQ,et al. Differential proteomic analysis of replanted Rehmannia glutinosa roots by iTRAQ reveals molecular mechanisms for formation of replant disease[J]. BMC Plant Biol,2017,17 (1):116. doi: 10.1186/s12870-017-1060-0
[12] Ramirez-Estrada K,Vidal-Limon H,Hidalgo D,Moyano E,Golenioswki M,et al. Elicitation,an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories[J]. Molecules,2016,21 (2):182. doi: 10.3390/molecules21020182
[13] Yuan YJ,Wei ZJ,Miao ZQ,Wu JC. Acting paths of elicitors on Taxol biosynthesis pathway and their synergistic effect[J]. Biochem Eng J,2002,10 (2):77−83. doi: 10.1016/S1369-703X(01)00159-0
[14] Bae KH,Choi YE,Shin CG,Kim YY,Kim YS. Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng C. A. Meyer) adventitious root cultures[J]. Biotechnol Lett,2006,28 (15):1163−1166. doi: 10.1007/s10529-006-9071-1
[15] Chen WH,Xu CM,Zeng JL,Zhao B,Wang XD,Wang YC. Improvement of echinacoside and acteoside production by two-stage elicitation in cell suspension culture of Cistanche deserticola[J]. World J Microbiol Biotechnol,2007,23 (10):1451−1458. doi: 10.1007/s11274-007-9389-4
[16] 王尧龙,黄璐琦,袁媛,查良平. 药用植物转录组研究进展[J]. 中国中药杂志,2015,40(11):2055−2061. Wang YL,Huang LQ,Yuan Y,Zha LP. Research advances on analysis of medicinal plants transcriptome[J]. China Journal of Chinese Materia Medica,2015,40 (11):2055−2061.
[17] Kumar D,Hazra S,Datta R,Chattopadhyay S. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA,ethylene and GSH against combined cold and osmotic stress[J]. Sci Rep,2016,6:36867. doi: 10.1038/srep36867
[18] Li C,Tao RF,Li Y,Duan MH,Xu JH. Transcriptome analysis of the thermosensitive genic male-sterile line provides new insights into fertility alteration in rice (Oryza sativa)[J]. Genomics,2020,112 (3):2119−2129. doi: 10.1016/j.ygeno.2019.12.006
[19] Wu WZ,Yang HB,Xing P,Dong Y,Shen J,et al. Comparative transcriptome analysis revealed the freezing tolerance signaling events in winter rapeseed (Brassica rapa L. )[J]. Front Genet,2022,13:871825. doi: 10.3389/fgene.2022.871825
[20] Chang YJ,Wang MZ,Li J,Lu SF. Transcriptomic analysis reveals potential genes involved in tanshinone biosynthesis in Salvia miltiorrhiza[J]. Sci Rep,2019,9 (1):14929. doi: 10.1038/s41598-019-51535-9
[21] Zhao MZ,Lin YP,Wang YF,Li XY,Han YL,et al. Transcriptome analysis identifies strong candidate genes for ginsenoside biosynthesis and reveals its underlying molecular mechanism in Panax ginseng C. A. Meyer[J]. Sci Rep,2019,9 (1):615. doi: 10.1038/s41598-018-36349-5
[22] Li QQ,Liu CB,Huang CY,Wang MF,Long T,et al. Transcriptome and metabonomics analysis revealed the molecular mechanism of differential metabolite production of Dendrobium nobile under different epiphytic patterns[J]. Front Plant Sci,2022,13:868472. doi: 10.3389/fpls.2022.868472
[23] Ma LG,Dong CM,Song C,Wang XL,Zheng XK,et al. De novo genome assembly of the potent medicinal plant Rehmannia glutinosa using nanopore technology[J]. Comput Struct Biotechnol J,2021,19:3954−3963. doi: 10.1016/j.csbj.2021.07.006
[24] Saimaru H,Orihara Y. Biosynthesis of acteoside in cultured cells of Olea europaea[J]. J Nat Med,2010,64 (2):139−145. doi: 10.1007/s11418-009-0383-z
[25] 周延清,邵露营,郭萌萌,朱佳琳. 地黄C3H基因的克隆及生物信息学分析[J]. 广西植物,2020,40(9):1281−1287. doi: 10.11931/guihaia.gxzw201907060 Zhou YQ,Shao LY,Guo MM,Zhu JL. Cloning and bioinformatics analysis of C3H gene in Rehmannia glutinosa[J]. Guihaia,2020,40 (9):1281−1287. doi: 10.11931/guihaia.gxzw201907060
[26] 李欣容,智惊宇,杨超飞,李铭铭,左鑫,等. 地黄毛蕊花糖苷合酶基因的克隆、亚细胞定位与表达特性分析[J]. 中草药,2020,51(18):4739−4746. doi: 10.7501/j.issn.0253-2670.2020.18.018 Li XR,Zhi JY,Yang CF,Li MM,Zuo X,et al. Cloning,subcellular location and expression analysis of an acteoside synthase gene from Rehmannia glutinosa[J]. Chinese Traditional and Herbal Drugs,2020,51 (18):4739−4746. doi: 10.7501/j.issn.0253-2670.2020.18.018
[27] Yang YH,Yang MR,Zhu JY,Dong KW,Yi YJ,et al. Functional characterization of tyrosine decarboxylase genes that contribute to acteoside biosynthesis in Rehmannia glutinosa[J]. Planta,2022,255 (3):64. doi: 10.1007/s00425-022-03849-8
[28] Wang FQ,Li XR,Zuo X,Li MM,Miao CY,et al. Transcriptome-wide identification of WRKY transcription factor and functional characterization of RgWRKY37 involved in acteoside biosynthesis in Rehmannia glutinosa[J]. Front Plant Sci,2021,12:739853. doi: 10.3389/fpls.2021.739853



 下载:
下载: