Physiological and metabolomic analysis of Plantago asiatica L. in response to cadmium stress
-
摘要:
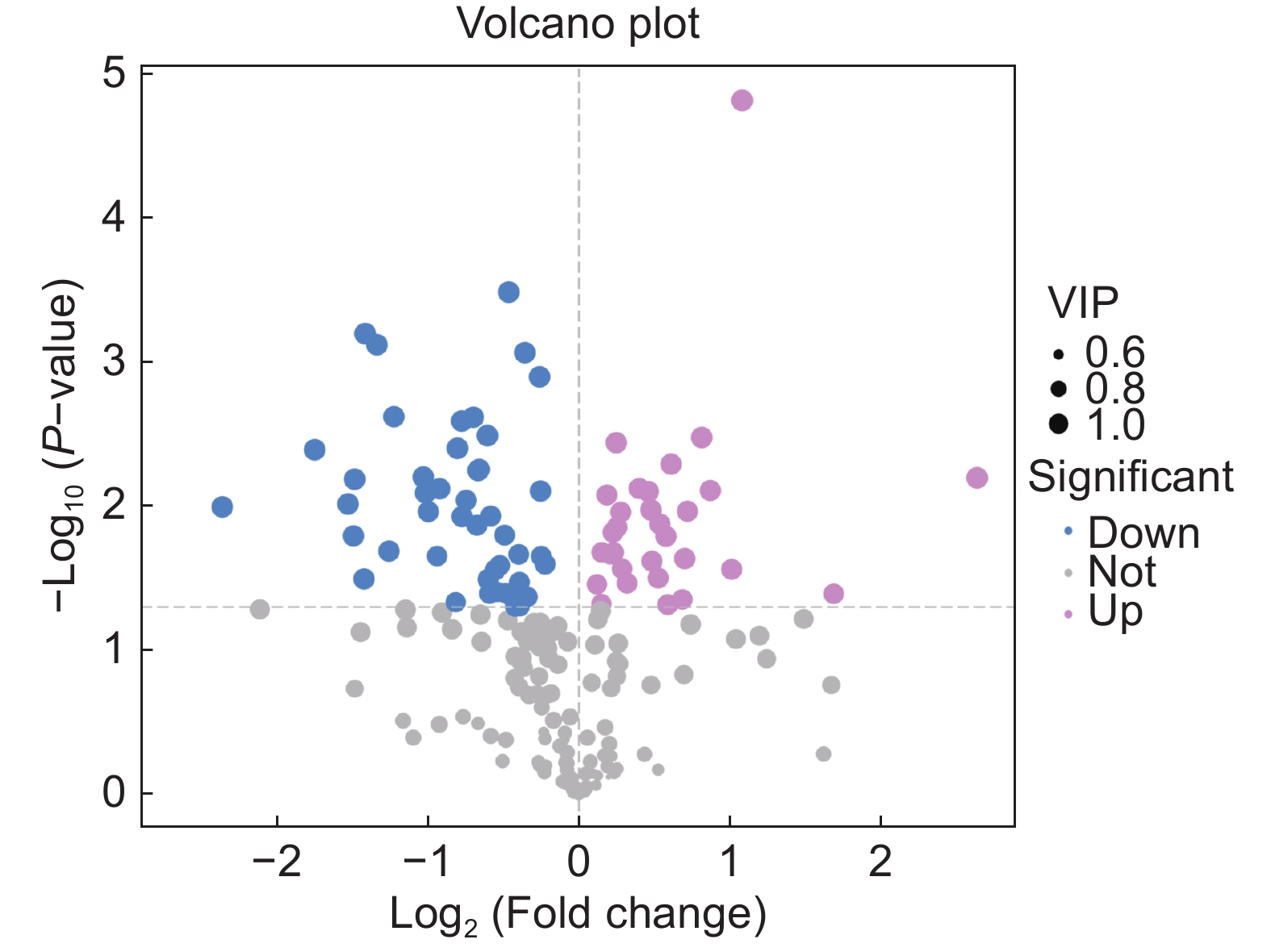
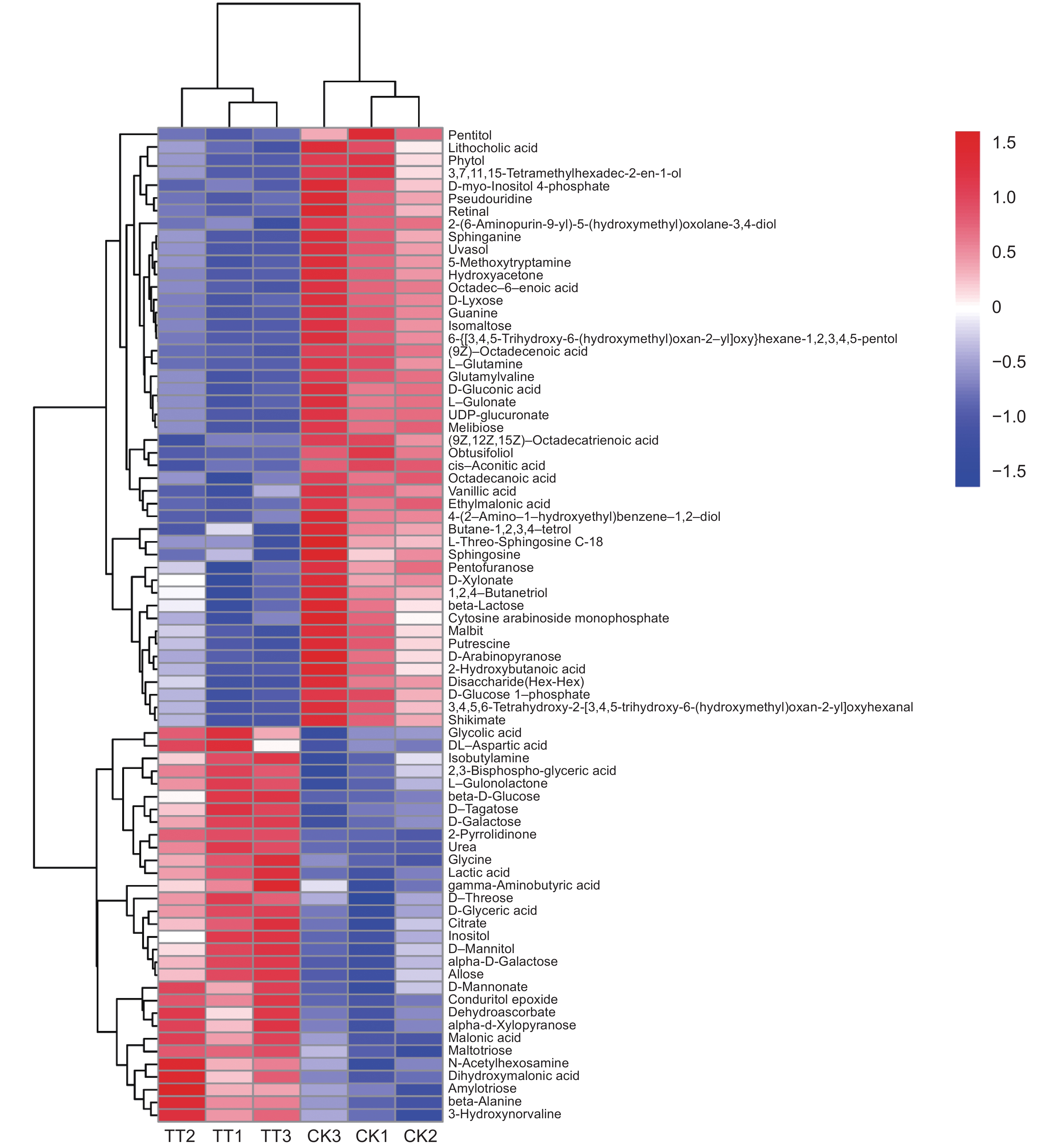
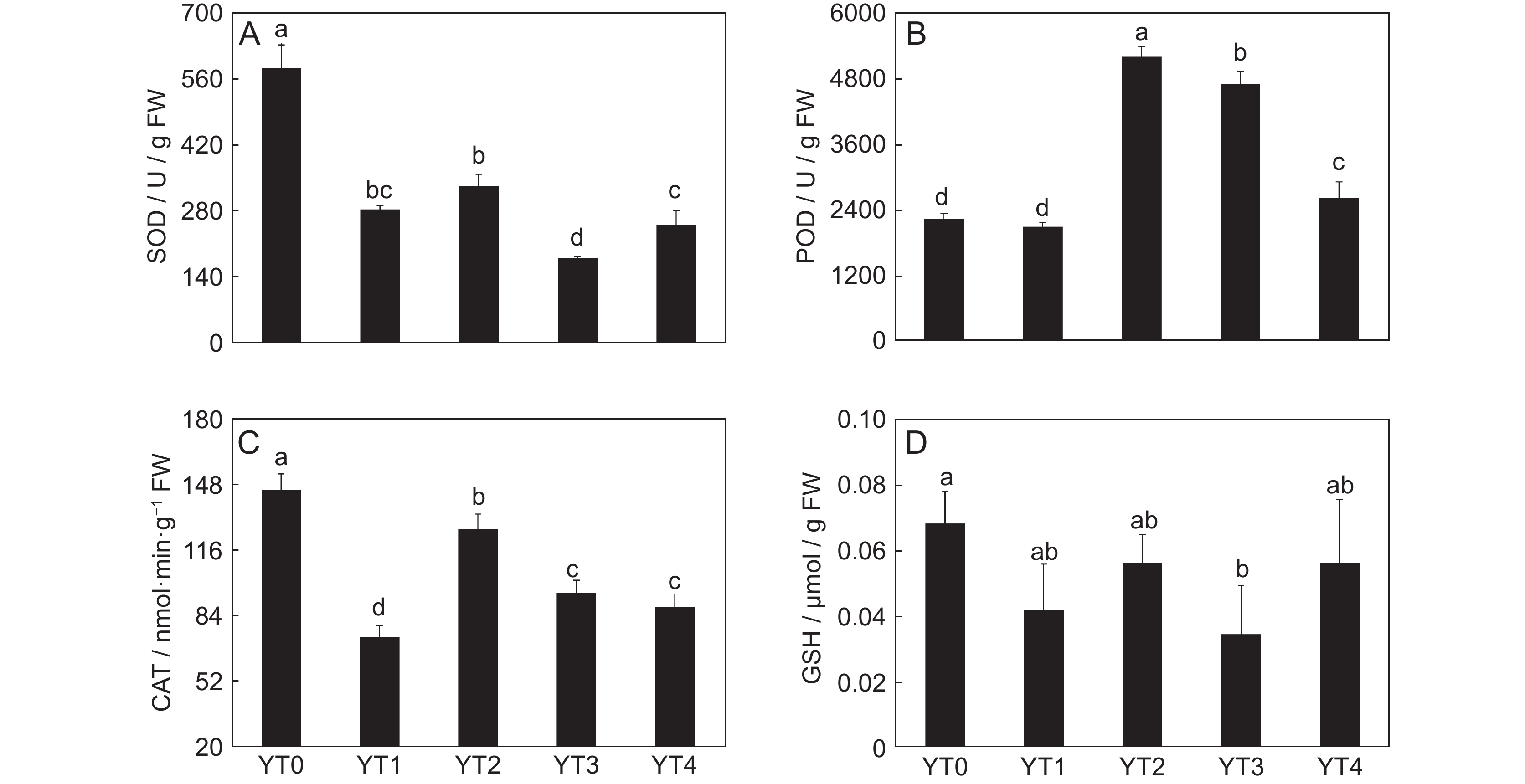
本研究测定了镉(Cd)胁迫下车前(Plantago asiatica L.)超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)的活性以及还原型谷胱甘肽(GSH)的含量,以探明车前响应Cd胁迫的生理机制,并进一步利用代谢组学方法筛选差异代谢物,并进行代谢通路富集分析。结果显示,随着Cd浓度的升高,SOD和CAT活性显著下降,POD活性显著上升,且总体呈先上升后下降的趋势,而GSH含量没有显著变化。50 mg/kg Cd胁迫组(YT3)与对照组(YT0)之间的显著差异代谢物有78个,其中31个上调,47个下调,且差异代谢物主要集中在糖类和氨基酸类。差异代谢物主要富集到20条通路中,包括3条糖代谢途径和3条脂类代谢途径。
Abstract:In this study, the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) and content of reduced glutathione (GSH) in Plantago asiatica L. under cadmium (Cd) stress were determined to explore the biological mechanisms of P. asiatica in response to such stress. Metabolomics was further used to screen differential metabolites, and metabolic pathway enrichment analysis was conducted. Results showed that with the increase in Cd concentration, the activities of SOD and CAT decreased significantly, POD activity increased significantly, with an overall trend of first increasing and then decreasing. GSH content showed no significant differences. There were 78 metabolites showing significant differences between the 50 mg/kg Cd stress group (YT3) and control group (YT0), including 31 up-regulated and 47 down-regulated metabolites, mainly carbohydrates and carboxylic acids. The differential metabolites were mainly enriched in 20 pathways, including three glucose metabolism pathways and three lipid metabolism pathways.
-
Keywords:
- Plantago asiatica /
- Cd stress /
- Physiological indexes /
- Metabonomics
-
1 1)如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。 -
图 1 车前在Cd胁迫下超氧化物歧化酶SOD(A)、过氧化物酶POD(B)、过氧化氢酶CAT(C)活性和还原型谷胱甘肽GSH含量(D)的变化
YT0、YT1、YT2、YT3和YT4分别表示对照组及3、10、50、100 mg/kg Cd胁迫组。不同小写字母表示在0.05水平显著差异。
Figure 1. Changes in SOD (A), POD (B), CAT (C), and GSH (D) in Plantago asiatica under Cd stress
YT0, YT1, YT2, YT3, and YT4 represent CK, 3, 10, 50, and 100 mg/kg Cd stress groups, respectively. Different lowercase letters indicate significant differences at 0.05 level.
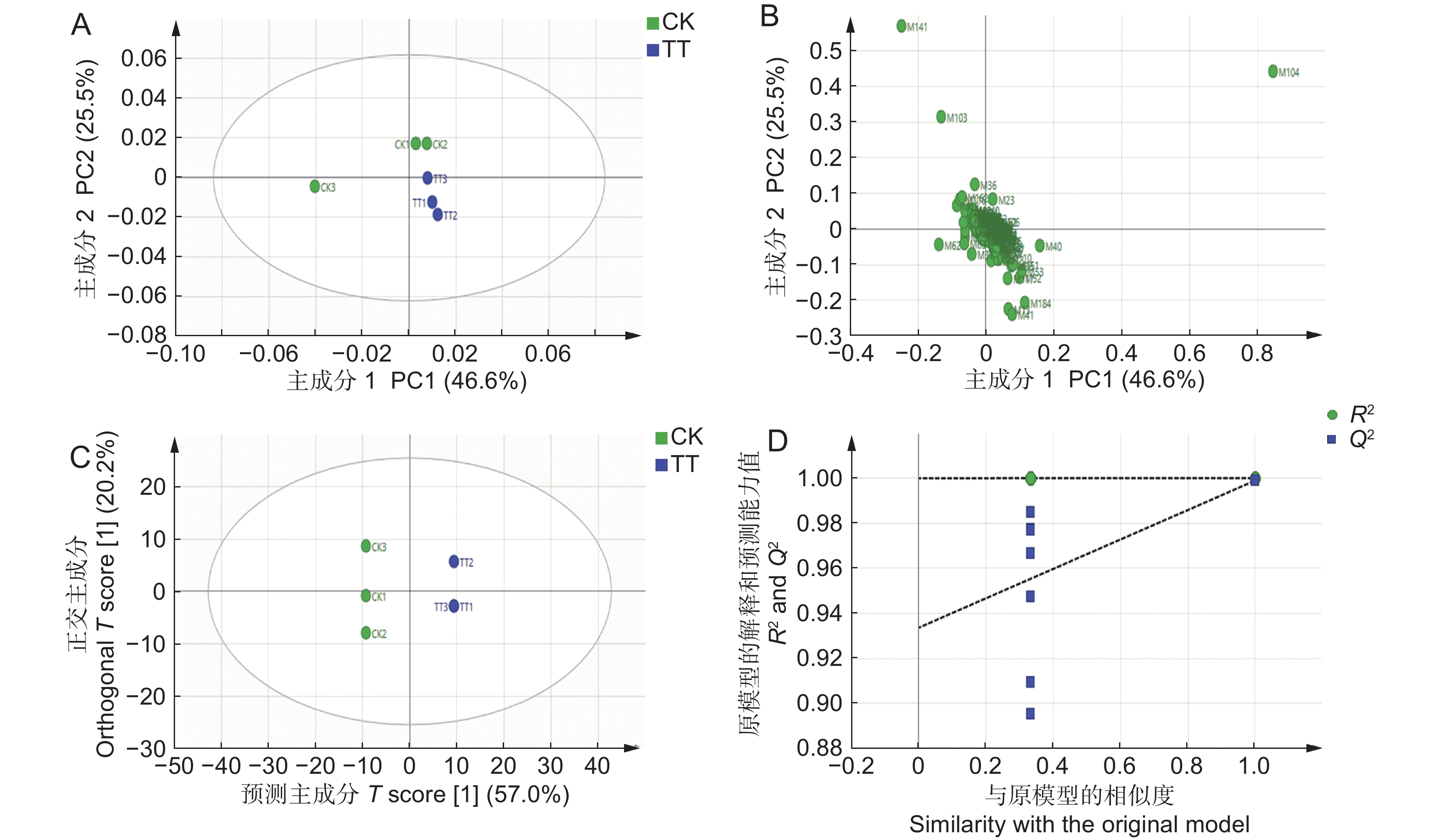
图 2 PCA得分散点图(A)、PCA载荷图(B)、OPLS-DA得分散点图(C)以及OPLS-DA模型的置换检验结果(D)
CK 和 TT 分别表示对照组和 50 mg/kg Cd 胁迫组。
Figure 2. Scatter plot of PCA scores (A), PCA load plot (B), scatter plot of OPLS-DA (C), and OPLS-DA model permutation test results (D)
CK and TT represent control and 50 mg/kg Cd stress groups, respectively.
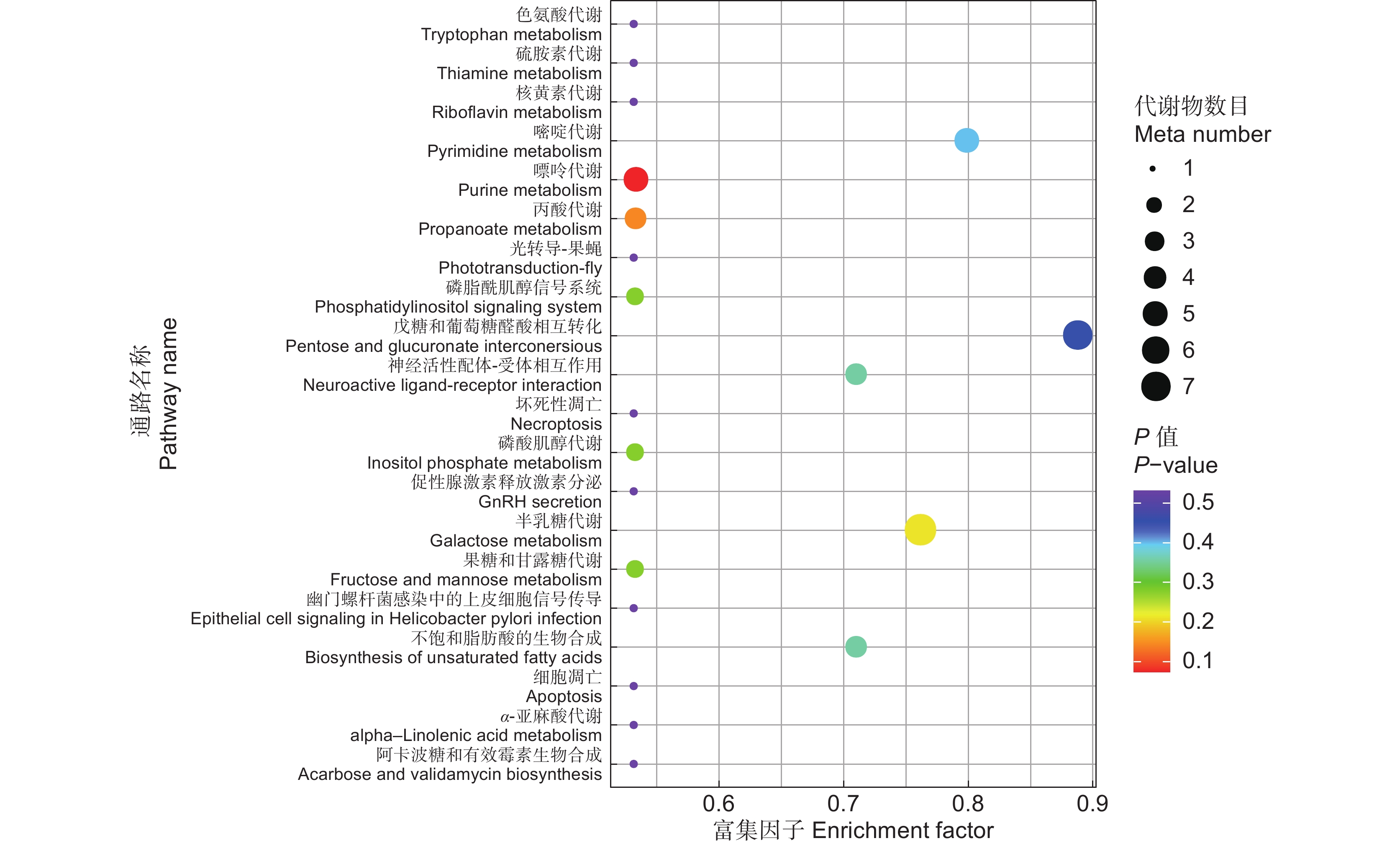
表 1 KEGG 通路富集详情
Table 1 KEGG pathway enrichment
KEGG 通路
KEGG pathway代谢物数目
Number of metabolites通路 ID
Pathway IDP 值
P-value化合物
Compound嘌呤代谢 4 map00230 0.07 cpd:C00037甘氨酸
cpd:C00242鸟嘌呤
cpd:C00064 L-谷氨酰胺
cpd:C00086尿素丙酸代谢 3 map00640 0.14 cpd:C05984 2-羟基丁酸
cpd:C05235 羟基丙酮
cpd:C00099 β-丙氨酸半乳糖代谢 7 map00052 0.21 cpd:C00984 α-D-半乳糖
cpd:C00103 D-葡萄糖1-磷酸
cpd:C00795 D-塔格糖
cpd:C00137肌醇
cpd:C05400蜜二糖
cpd:C05400蜜二糖
cpd:C00984 α-D-半乳糖磷酸肌醇代谢 2 map00562 0.28 cpd:C03546 D-肌醇4-磷酸
cpd:C00137肌醇磷脂酰肌醇信号系统 2 map04070 0.28 cpd:C03546 D-肌醇4-磷酸
cpd:C00137肌醇果糖和甘露糖代谢 2 map00051 0.28 cpd:C01487 D-阿洛糖
cpd:C00392甘露糖醇不饱和脂肪酸的生物合成 3 map01040 0.36 cpd:C00712油酸
cpd:C06427 α-亚麻酸
cpd:C01530硬脂酸神经活性配体-受体相互作用 3 map04080 0.36 cpd:C00037甘氨酸
cpd:C00099 β-丙氨酸
cpd:C00334 4-氨基丁酸嘧啶代谢 4 map00240 0.40 cpd:C00064 L-谷氨酰胺
cpd:C02067假尿苷
cpd:C00086尿素
cpd:C00099 β-丙氨酸戊糖和葡萄糖醛酸相互转化 6 map00040 0.45 cpd:C00103 D-葡萄糖-1-磷酸
cpd:C00514 D-甘露酸盐
cpd:C00502 D-木糖酸盐
cpd:C00800 L-古洛糖酸
cpd:C00474核糖醇
cpd:C00167 UDP-葡萄糖醛酸色氨酸代谢 1 map00380 0.53 cpd:C05659 5-甲氧基色胺 硫胺素代谢 1 map00730 0.53 cpd:C00037甘氨酸 核黄素代谢 1 map00740 0.53 cpd:C00474核糖醇 光转导-果蝇 1 map04745 0.53 cpd:C01530硬脂酸 坏死性凋亡 1 map04217 0.53 cpd:C00319鞘氨醇 促性腺激素释放激素分泌 1 map04929 0.53 cpd:C00334 4-氨基丁酸 幽门螺杆菌感染中的上皮细胞信号传导 1 map05120 0.53 cpd:C00086尿素 细胞凋亡 1 map04210 0.53 cpd:C00319鞘氨醇 α-亚麻酸代谢 1 map00592 0.53 cpd:C06427 α-亚麻酸 阿卡波糖和有效霉素生物合成 1 map00525 0.53 cpd:C00103 D-葡萄糖 1-磷酸 -
[1] 张雪芹,曲玮,梁敬钰. 车前草化学成分和药理作用研究进展[J]. 海峡药学,2013,25(11):1−8. Zhang XQ,Qu W,Liang JY. Research progresses on chemical constituents and pharmacological activities of Plantago spp.[J]. Strait Pharmaceutical Journal,2013,25 (11):1−8.
[2] 国家药典委员会. 中华人民共和国药典: 一部[M]. 北京: 中国医药科技出版社, 2020: 69−70. [3] Cui L,Wang XN,Li J,Gao XY,Zhang JW,Liu ZT. Ecological and health risk assessments and water quality criteria of heavy metals in the Haihe River[J]. Environ Pollut,2021,290:117971. doi: 10.1016/j.envpol.2021.117971
[4] Mahvi AH,Eslami F,Baghani AN,Khanjani N,Yaghmaeian K,Mansoorian HJ. Heavy metal pollution status in soil for different land activities by contamination indices and ecological risk assessment[J]. Int J Environ Sci Technol,2022,19 (8):7599−7616. doi: 10.1007/s13762-022-03960-z
[5] Bahloul M. Pollution characteristics and health risk assessment of heavy metals in dry atmospheric deposits from Sfax solar saltern area in southeast of Tunisia[J]. J Environ Health Sci Eng,2019,17 (2):1085−1105. doi: 10.1007/s40201-019-00423-5
[6] 环境保护部, 国土资源部. 全国土壤污染状况调查公报[EB/OL]. (2014-04-17) [2023-03-02]. http://www.gov.cn/foot/site1/20140417/782bcb88840814ba158d01.pdf. [7] 安婷婷,黄帝,王浩,张一,陈应龙. 植物响应镉胁迫的生理生化机制研究进展[J]. 植物学报,2021,56(3):347−362. doi: 10.11983/CBB20160 An TT,Huang D,Wang H,Zhang Y,Chen YL. Research advances in plant physiological and biochemical mechanisms in response to cadmium stress[J]. Chinese Bulletin of Botany,2021,56 (3):347−362. doi: 10.11983/CBB20160
[8] 文珂,刘文胜,赵运林. 重金属污染区与非污染区平车前生物量分配的比较[J]. 中南林业科技大学学报,2018,38(1):94−98. Wen K,Liu WS,Zhao YL. Comparisons of biomass allocation between Plantago depressa plants growing in heavy-metal polluted and nonpolluted soils[J]. Journal of Central South University of Forestry & Technology,2018,38 (1):94−98.
[9] Feng Z,Ji SY,Ping JF,Cui D. Recent advances in metabolomics for studying heavy metal stress in plants[J]. Trends Analyt Chem,2021,143:116402. doi: 10.1016/j.trac.2021.116402
[10] Ghatak A,Chaturvedi P,Weckwerth W. Metabolomics in plant stress physiology[J]. Adv Biochem Eng Biotechnol,2018,164:187−236.
[11] 刘荣鹏, 盛莎莎, 王晓云, 袁俊. 江西道地药用植物车前镉富集特点及其响应镉胁迫的转录组分析[J/OL]. 分子植物育种, 2023: 1−12. (2023-03-02). https://kns.cnki.net/kcms/detail//46.1068.s.20230228.1513.013.html. Liu RP, Sheng SS, Wang XY, Yuan J. Characteristics of cadmium enrichment of Jiangxi Daodi medicinal plant Plantago asiatica L. and its transcriptome analysis in response to cadmium stress[J/OL]. Molecular Plant Breeding, 2023: 1−12.(2023-03-02). https://kns.cnki.net/kcms/detail//46.1068.s.20230228.1513.013.html.
[12] Yuan J,Liu R,Sheng S,Fu H,Wang X. Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance[J]. Open Life Sci,2022,17 (1):1347−1359. doi: 10.1515/biol-2022-0501
[13] 张凤,陈伟. 代谢组学在植物逆境生物学中的研究进展[J]. 生物技术通报,2021,37(8):1−11. Zhang F,Chen W. Research progress of metabolomics in plant stress biology[J]. Biotechnology Bulletin,2021,37 (8):1−11.
[14] 郭晋敏, 杨升, 陈秋夏, 刘星, 刘双双, 等. 极端低温胁迫下秋茄LC-MS代谢组学分析[J/OL]. 分子植物育种, 2021: 1−16. (2021-09-09). http://kns.cnki.net/kcms/detail/46.1068.S.20210909.1512.022.html. Guo JM, Yang S, Chen QX, Liu X, Liu SS, et al. Analysis of LC-MS metabolomics of Kandelia obovata under extreme low temperature stress[J/OL]. Molecular Plant Breeding, 2021: 1−16. (2021-09-09). http://kns.cnki.net/kcms/detail/46.1068.S.20210909.1512.022.html.
[15] 原静静,孙晓琛,栗锦鹏,杜弢,王惠珍. 基于LC-MS的干旱胁迫下党参代谢组学分析[J]. 中国实验方剂学杂志,2021,27(23):145−152. Yuan JJ,Sun XC,Li JP,Du T,Wang HZ. Metabolomics analysis of Codonopsis pilosula under drought stress based on LC-MS[J]. Chinese Journal of Experimental Traditional Medical Formulae,2021,27 (23):145−152.
[16] 朱建峰, 张会龙, 杨秀艳, 武海雯, 张华新. 盐胁迫下白榆种子萌发期代谢组学分析[J/OL]. 分子植物育种, 2021: 1-15. (2021-06-09). http://kns.cnki.net/kcms/detail/46.1068.S.20210609.1326.016.html. Zhu JF, Zhang HL, Yang XY, Wu HW, Zhang HX. Metabonomic analysis of seed germination period of Ulmus pumila under salt stress[J/OL]. Molecular Plant Breeding, 2021: 1−15. (2021-06-09). http://kns.cnki.net/kcms/detail/46.1068.S.20210609.1326.016.html.
[17] Hrydziuszko O,Viant MR. Missing values in mass spectrometry based metabolomics:an undervalued step in the data processing pipeline[J]. Metabolomics,2012,8 (S1):161−174. doi: 10.1007/s11306-011-0366-4
[18] Dunn WB,Broadhurst D,Begley P,Zelena E,Francis-Mcintyre S,et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry[J]. Nat Protoc,2011,6 (7):1060−1083. doi: 10.1038/nprot.2011.335
[19] Jolliffe IT,Cadima J. Principal component analysis:a review and recent developments[J]. Phil Trans Roy Soc A Math Phys Eng Sci,2016,374 (2065):20150202.
[20] Wiklund S,Johansson E,Sjöström L,Mellerowicz EJ,Edlund U,et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models[J]. Anal Chem,2008,80 (1):115−122. doi: 10.1021/ac0713510
[21] Robotti E, Marengo E. Chemometric multivariate tools for candidate biomarker identification: LDA, PLS-DA, SIMCA, Ranking-PCA[M]//Marengo E, Robotti E, eds. 2-D PAGE Map Analysis: Methods and Protocols. New York: Humana, 2016: 237−267.
[22] Wishart DS,Feunang YD,Marcu A,Guo AC,Liang K,et al. HMDB 4.0:the human metabolome database for 2018[J]. Nucleic Acids Res,2018,46 (D1):D608−D617. doi: 10.1093/nar/gkx1089
[23] Hattori M,Tanaka N,Kanehisa M,Goto S. SIMCOMP/SUBCOMP:chemical structure search servers for network analyses[J]. Nucleic Acids Res,2010,38 (S2):W652−W656.
[24] Kanehisa M,Sato Y,Kawashima M,Furumichi M,Tanabe M. KEGG as a reference resource for gene and protein annotation[J]. Nucleic Acids Res,2016,44 (D1):D457−D462. doi: 10.1093/nar/gkv1070
[25] Xia JG,Sinelnikov IV,Han B,Wishart DS. MetaboAnalyst 3.0-making metabolomics more meaningful[J]. Nucleic Acids Res,2015,43 (W1):W251−W257. doi: 10.1093/nar/gkv380
[26] Saccenti E,Hoefsloot HCJ,Smilde AK,Westerhuis JA,Hendriks MMWB. Reflections on univariate and multivariate analysis of metabolomics data[J]. Metabolomics,2014,10 (3):361−374. doi: 10.1007/s11306-013-0598-6
[27] Das K,Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants[J]. Front Environ Sci,2014,2:53.
[28] Zhou XR,Joshi S,Patil S,Khare T,Kumar V. Reactive oxygen,nitrogen,carbonyl and sulfur species and their roles in plant abiotic stress responses and tolerance[J]. J Plant Growth Regul,2022,41 (1):119−142. doi: 10.1007/s00344-020-10294-y
[29] 全芮萍,陈建福,张蕾,许明志,杨瑞芳,等. 抗氧化酶和植物螯合肽对苎麻重金属Cd胁迫的应答[J]. 热带作物学报,2022,43(5):1023−1031. doi: 10.3969/j.issn.1000-2561.2022.05.017 Quan RP,Chen JF,Zhang L,Xu MZ,Yang RF,et al. Responses of ramie to antioxidant enzymes and plant chelating peptides to Cd stress[J]. Chinese Journal of Tropical Crops,2022,43 (5):1023−1031. doi: 10.3969/j.issn.1000-2561.2022.05.017
[30] 张金钰. 糯玉米苗期镉胁迫下转录组和代谢组分析[D]. 广州: 仲恺农业工程学院, 2020: 78. [31] Wang JC,Chen XF,Chu SH,You YM,Chi YW,et al. Comparative cytology combined with transcriptomic and metabolomic analyses of Solanum nigrum L. in response to Cd toxicity[J]. J Hazard Mater,2022,423:127168. doi: 10.1016/j.jhazmat.2021.127168
[32] 曹莹,李建东,赵天宏,郭伟. 镉胁迫对玉米生理生化特性的影响[J]. 农业环境科学学报,2007,26(S1):8−11. Cao Y,Li JD,Zhao TH,Guo W. Effects of Cd stress on physiological and biochemical traits of maize[J]. Journal of Agro-Environment Science,2007,26 (S1):8−11.
[33] 朱润华,贺忠群,王海霞,白胜,阳圣莹,蒋浩宏. 镉胁迫处理对水培苦苣幼苗生理响应及叶片超微结构的影响[J]. 西南农业学报,2021,34(6):1302−1308. Zhu RH,He ZQ,Wang HX,Bai S,Yang SY,Jiang HH. Effects of cadmium stress on physiological response and leaf ultrastructure of hydroponic Cichorium endivia L. seedling[J]. Southwest China Journal of Agricultural Sciences,2021,34 (6):1302−1308.
[34] 黄东华,麦淑华,仇曙,陈大清. 镉对堇叶碎米荠生长生理特性的影响[J]. 湖北农业科学,2022,61(5):87−90. Huang DH,Mai SH,Qiu S,Chen DQ. Effects of cadmium on growth and physiological characteristics of Cardamine violifolia[J]. Hubei Agricultural Sciences,2022,61 (5):87−90.
[35] 卢倩云,曹宇棽,陈友明,晏琼. 镉胁迫下油菜毛状根的生理响应及铁钾含量[J]. 应用与环境生物学报,2018,24(6):1382−1389. Lu QY,Cao YS,Chen YM,Yan Q. The physiological response and iron and potassium contents in the hairy roots of Brassica rape L. under cadmium stress[J]. Chinese Journal of Applied and Environmental Biology,2018,24 (6):1382−1389.
[36] Zhang QW,Song XM,Bartels D. Enzymes and metabolites in carbohydrate metabolism of desiccation tolerant plant[J]. Proteomes,2016,4 (4):40. doi: 10.3390/proteomes4040040
[37] 胡雪萍. 水稻响应镉胁迫代谢组学研究[D]. 南昌: 南昌大学, 2019: 78. [38] 汪怡文. 转录组和代谢组整合分析冬小麦镉胁迫响应的关键代谢通路[D]. 武汉: 华中农业大学, 2021: 81. [39] 谭飘飘. 芥菜对镉胁迫的生理代谢响应及外源脯氨酸的调控作用研究[D]. 长沙: 中南林业科技大学, 2021: 105. [40] 胡立群,徐庆国. 植物非生物胁迫代谢组学研究进展[J]. 作物研究,2014,28(4):428−434. doi: 10.3969/j.issn.1001-5280.2014.04.24 Hu LQ,Xu QG. Review of current progress in the metabolomics for plant response to abiotic stress[J]. Crop Research,2014,28 (4):428−434. doi: 10.3969/j.issn.1001-5280.2014.04.24
[41] Yang QQ,Zhao DS,Liu QQ. Connections between amino acid metabolisms in plants:lysine as an example[J]. Front Plant Sci,2020,11:928. doi: 10.3389/fpls.2020.00928
[42] Hildebrandt TM. Synthesis versus degradation:directions of amino acid metabolism during Arabidopsis abiotic stress response[J]. Plant Mol Biol,2018,98 (1-2):121−135. doi: 10.1007/s11103-018-0767-0
[43] Rizhsky L,Liang HJ,Shuman J,Shulaev V,Davletova S,Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress[J]. Plant Physiol,2004,134 (4):1683−1696. doi: 10.1104/pp.103.033431
[44] 袁俊,盛莎莎,刘荣鹏,王晓云. 镉胁迫对丹参生理特性和代谢特征的影响[J]. 植物科学学报,2022,40(3):408−417. doi: 10.11913/PSJ.2095-0837.2022.30408 Yuan J,Sheng SS,Liu RP,Wang XY. Effects of cadmium on Physiological characteristics and metabolic profiles of Salvia miltiorrhiza Bunge[J]. Plant Science Journal,2022,40 (3):408−417. doi: 10.11913/PSJ.2095-0837.2022.30408
[45] Yuan J,Liu R,Sheng S,Fu H,Wang X. Untargeted LC-MS/MS-Based metabolomic profiling for the edible and medicinal plant Salvia miltiorrhiza under different levels of cadmium stress[J]. Front Plant Sci,2022,13:889370. doi: 10.3389/fpls.2022.889370
[46] Kaplan F,Kopka J,Haskell DW,Zhao W,Schiller KC,et al. Exploring the temperature-stress metabolome of Arabidopsis[J]. Plant Physiol,2004,136 (4):4159−4168. doi: 10.1104/pp.104.052142
[47] Sun XM,Zhang JX,Zhang HJ,Ni YW,Zhang Q,et al. The responses of Arabidopsis thaliana to cadmium exposure explored via metabolite profiling[J]. Chemosphere,2010,78 (7):840−845. doi: 10.1016/j.chemosphere.2009.11.045
[48] Broeckling CD,Huhman DV,Farag MA,Smith JT,May GD,et al. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism[J]. J Exp Bot,2005,56 (410):323−336. doi: 10.1093/jxb/eri058
[49] Parthasarathy A,Savka MA,Hudson AO. The synthesis and role of β-Alanine in plants[J]. Front Plant Sci,2019,10:921. doi: 10.3389/fpls.2019.00921
[50] Katahira R,Ashihara H. Dual function of pyrimidine metabolism in potato (Solanum tuberosum) plants:pyrimidine salvage and supply of β‐alanine to pantothenic acid synthesis[J]. Physiol Plant,2006,127 (1):38−43. doi: 10.1111/j.1399-3054.2006.00658.x
[51] 王佳钰,李萌,宋晓卉,李琦,齐秀芬,王兰兰. 代谢组学在植物重金属胁迫研究中的应用[J]. 生物化工,2020,6(2):128−132. doi: 10.3969/j.issn.2096-0387.2020.02.037 Wang JY,Li M,Song XH,Li Q,Qi XF,Wang LL. The application of metabonomics in study of plant under heavy metal stress[J]. Biological Chemical Engineering,2020,6 (2):128−132. doi: 10.3969/j.issn.2096-0387.2020.02.037
[52] Kim HU. Lipid metabolism in plants[J]. Plants,2020,9 (7):871. doi: 10.3390/plants9070871
[53] Zhao XC,Wei YL,Zhang JJ,Yang LY,Liu XY,et al. Membrane lipids’ metabolism and transcriptional regulation in maize roots under cold stress[J]. Front Plant Sci,2021,12:639132. doi: 10.3389/fpls.2021.639132
[54] 贾斌, 高龙飞, 张卫华, 勾峰, 李天杰, 等. 西瓜苗期干旱胁迫下的代谢组学分析[J/OL]. 分子植物育种, 2021: 1−15. (2021-11-20). http://kns.cnki.net/kcms/detail/46.1068.S.20211118.2148.007.html. Jia B, Gao LF, Zhang WH, Gou F, Li TJ, et al. Metabonomics analysis of watermelon seedlings under drought stress[J/OL]. Molecular Plant Breeding, 2021: 1−15. (2021-11-20). http://kns.cnki.net/kcms/detail/46.1068.S.20211118.2148.007.html.
[55] 方彦,曾秀存,马骊,孙柏林,武军艳,等. 低温胁迫下冬油菜陇油7号根部代谢组学分析[J]. 干旱地区农业研究,2021,39(4):80−85. doi: 10.7606/j.issn.1000-7601.2021.04.10 Fang Y,Zeng XC,Ma L,Sun BL,Wu JY,et al. Metabolomics profiling of Brassica campestris Longyou 7 roots under cold stress[J]. Agricultural Research in the Arid Areas,2021,39 (4):80−85. doi: 10.7606/j.issn.1000-7601.2021.04.10
[56] Sun L,Cao X,Tan C,Deng Y,Cai R,Peng X,et al. Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics[J]. Ecotox Environ Safe,2020,205:111152. doi: 10.1016/j.ecoenv.2020.111152
-
其他相关附件
-
DOCX格式
盛莎莎附表1 点击下载(29KB)
-



 下载:
下载: