Utilization strategies of inorganic nitrogen in submerged macrophytes
-
摘要:
沉水植物在水体生态系统中发挥着关键作用,能通过直接和间接途径缓解水体中的氮负荷,促进湖泊生态系统的良性运转。沉水植物在无机氮的利用策略上与陆生植物差异显著,为了适应水环境,沉水植物地上和地下部分能够同时获取环境中的氮,并将其进行向上或向下的运输。为了减少高浓度氮的毒害,沉水植物地上和地下部分在氮利用中存在一定的权衡关系。沉水植物氮同化的主要场所是叶片,主要通过谷氨酰胺合成酶/谷氨酸合成酶(GS /GOGAT)循环和谷氨酸脱氢酶(GDH)途径进行氮的同化。目前针对沉水植物的相关研究还远不及陆生植物,仅涉及到生理响应层面,仍需深入探索沉水植物氮素利用的机理,开发合适的遗传转化体系,并利用基因编辑等技术对基因功能进行验证,对关键蛋白质的结构与功能展开深入研究。
Abstract:As important components in aquatic ecosystems, submerged macrophytes can alleviate nitrogen load and improve the healthy operation of ecosystems through direct and indirect ways. Nitrogen utilization strategies of submerged macrophytes differ significantly from those of terrestrial plants. Submerged macrophytes can uptake nitrogen not only from the overlying water, but also from pore water in sediments via above-ground and below-ground parts, respectively. To adapt to the various changes in nitrogen content in aquatic environments, submerged macrophytes exhibit two directions of nitrogen translocation, namely acropetal and basipetal translocation. To avoid the toxicity caused by high nitrogen concentrations, a trade-off exists in nitrogen utilization between the above- and below-ground parts of submerged macrophytes. The GS/GOGAT cycle and GDH pathway are the primary pathways for nitrogen assimilation in submerged macrophytes. Currently, research on submerged macrophytes lags far behind that on terrestrial plants. Further exploration of the mechanisms underlying nitrogen utilization in submerged macrophytes is still needed and a suitable genetic transformation system of submerged macrophytes is required. Molecular technologies such as gene editing can be used to identify gene function, which should promote further studies on the structure and function of key proteins.
-
-
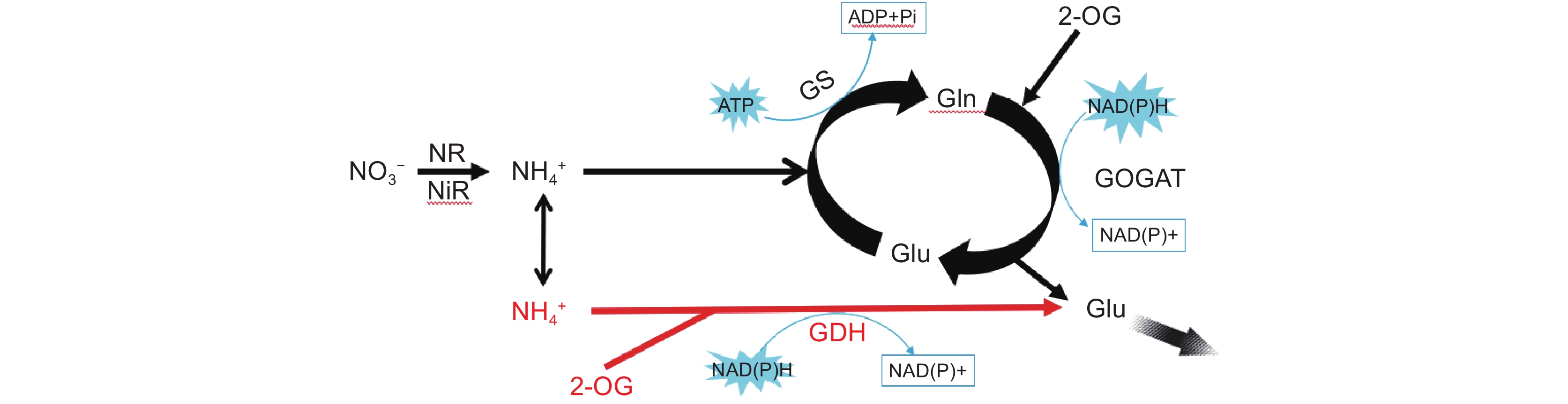
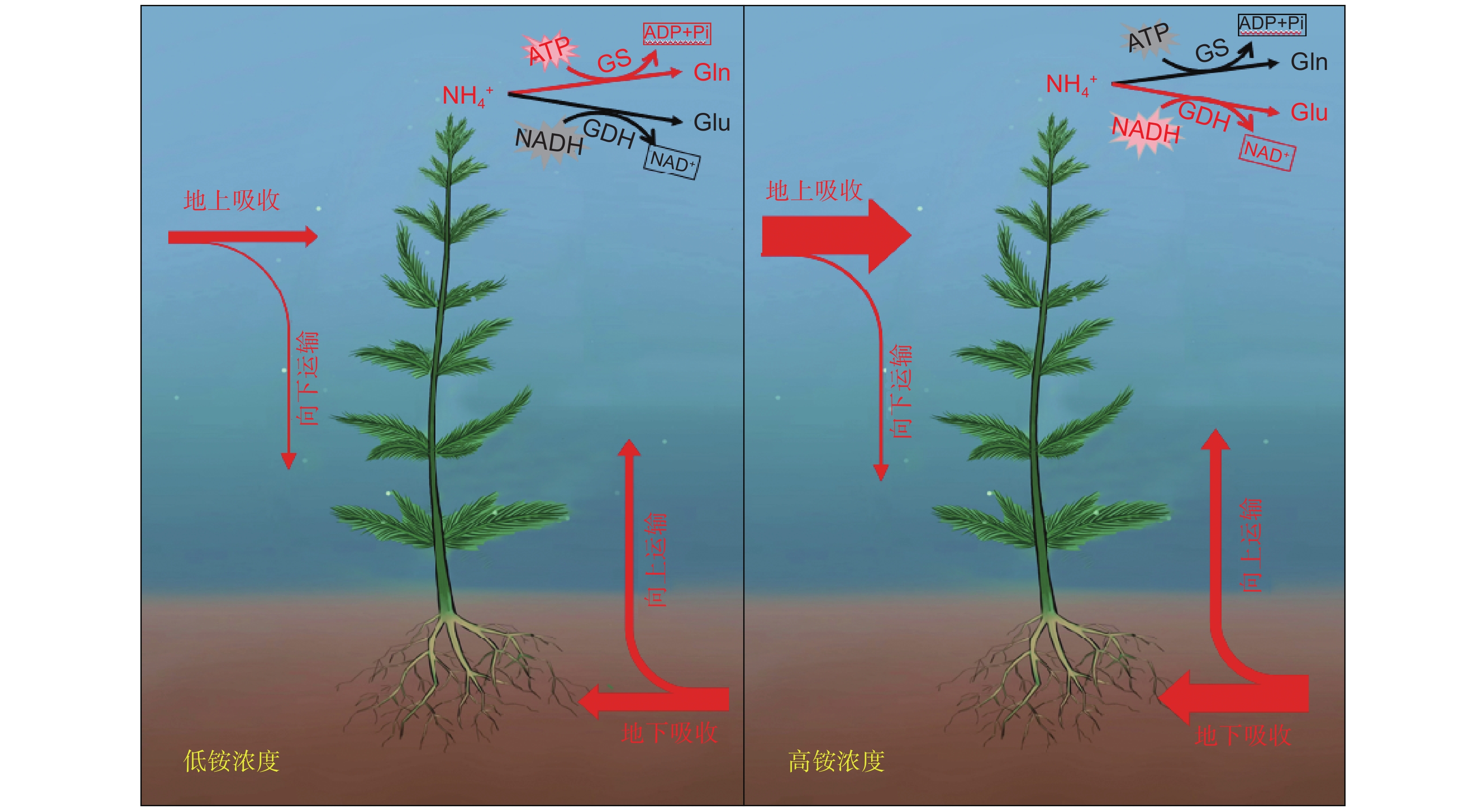
图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])
箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。
Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])
Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.
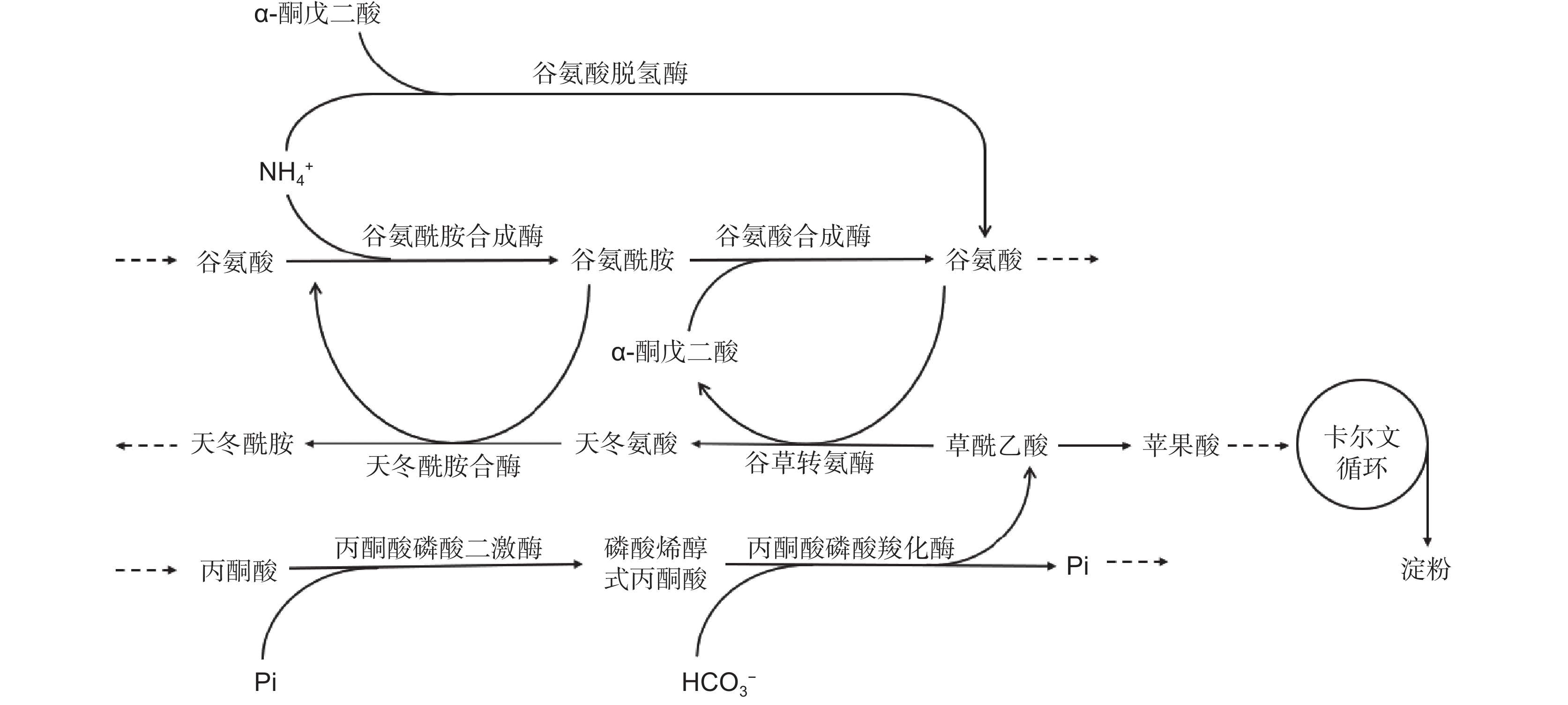
图 2 植物无机氮同化路径
GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。
Figure 2. Inorganic nitrogen assimilation pathways in plants
GS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.
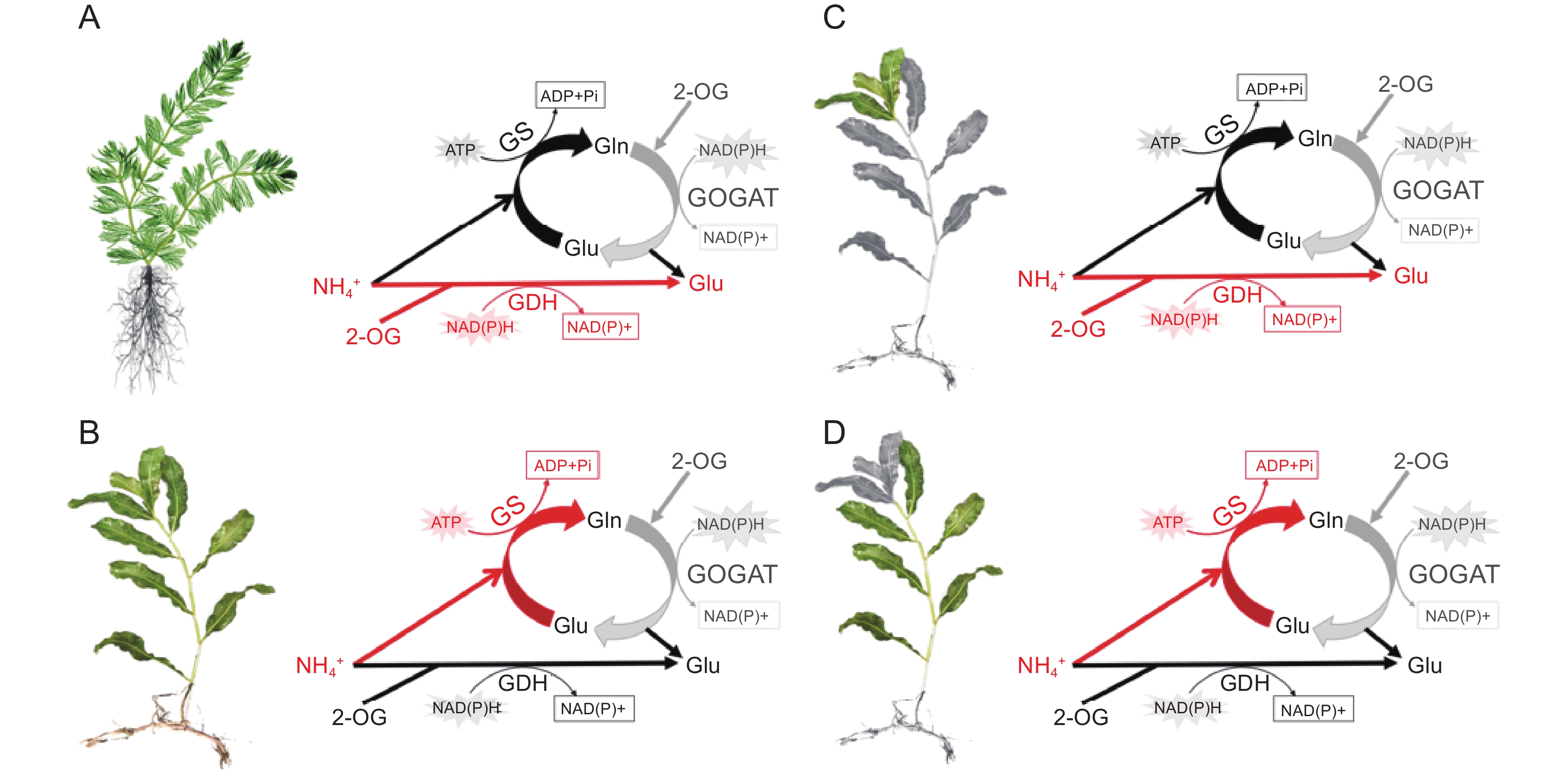
图 3 不同适应能力及不同发育时期沉水植物的无机氮同化路径(改自Xian等[46, 68]和Ochieng等[69])
A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。
Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])
A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.
-
[1] Philbrick CT,Les DH. The evolution of aquatic plants:an introduction and tribute to Cyril Duncan Sculthorpe,author of the biology of aquatic vascular plants[J]. Aquat Bot,1993,44(2-3):101−104. doi: 10.1016/0304-3770(93)90067-7
[2] Carpenter SR,Lodge DM. Effects of submersed macrophytes on ecosystem processes[J]. Aquat Bot,1986,26:341−370. doi: 10.1016/0304-3770(86)90031-8
[3] 严雪,于丹,李永科. CO2浓度升高对沉水克隆植物生长速率及营养元素积累的影响[J]. 植物生态学报,2003,27(4):435−440. doi: 10.3321/j.issn:1005-264X.2003.04.001 Yan X,Yu D,Li YK. Response of growth rate and nutrient elements accumulation in submerged clonal plants macrophyte under elevated CO2[J]. Acta Phytoecologica Sinica,2003,27(4):435−440. doi: 10.3321/j.issn:1005-264X.2003.04.001
[4] Kiba T,Krapp A. Plant nitrogen acquisition under low availability:regulation of uptake and root architecture[J]. Plant Cell Physiol,2016,57(4):707−714. doi: 10.1093/pcp/pcw052
[5] Kusano M,Fukushima A,Redestig H,Saito K. Metabolomic approaches toward understanding nitrogen metabolism in plants[J]. J Exp Bot,2011,62(4):1439−1453. doi: 10.1093/jxb/erq417
[6] Özkan K,Jeppesen E,Johansson LS,Beklioglu M. The response of periphyton and submerged macrophytes to nitrogen and phosphorus loading in shallow warm lakes:a mesocosm experiment[J]. Freshwater Biol,2010,55(2):463−475. doi: 10.1111/j.1365-2427.2009.02297.x
[7] Toniciolli Rigueto CV,Rosseto M,Loss RA,dos Santos Richards NSP,Dettmer A,Pizzutti IR. Gelatin-based polymeric films for applications in food packaging:an overview of advances,challenges,and perspectives[J]. Ciênc Rural,2023,53(2):e20210679.
[8] Siefert A,Violle C,Chalmandrier L,Albert CH,Taudiere A,et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities[J]. Ecol Lett,2015,18(12):1406−1419. doi: 10.1111/ele.12508
[9] Barker T,Hatton KO,Connor L,Moss B. Effects of nitrate load on submerged plant biomass and species richness:results of a mesocosm experiment[J]. Fundam Appl Limnol,2008,173(2):89−100. doi: 10.1127/1863-9135/2008/0173-0089
[10] Pan YD,Birdsey RA,Fang JY,Houghton R,Kauppi PE,et al. A large and persistent carbon sink in the world’s forests[J]. Science,2011,333(6045):988−993. doi: 10.1126/science.1201609
[11] Olsen S,Chan F,Li W,Zhao ST,Søndergaard M,Jeppesen E. Strong impact of nitrogen loading on submerged macrophytes and algae:a long-term mesocosm experiment in a shallow Chinese lake[J]. Freshwater Biol,2015,60(8):1525−1536. doi: 10.1111/fwb.12585
[12] 常诏峰. 高原沉水植物对铵盐的生理响应研究[D]. 拉萨:西藏大学,2021:52−55. [13] Mokhele B,Zhan XJ,Yang GZ,Zhang XL. Review:nitrogen assimilation in crop plants and its affecting factors[J]. Can J Plant Sci,2012,92(3):399−405. doi: 10.4141/cjps2011-135
[14] Peuke AD,Jeschke WD,Hartung W. Foliar application of nitrate or ammonium as sole nitrogen supply in Ricinus communis. Ⅱ. The flows of cations,chloride and abscisic acid[J]. New Phytol,1998,140(4):625−636. doi: 10.1046/j.1469-8137.1998.00304.x
[15] Rees DC,Howard JB. Nitrogenase:standing at the crossroads[J]. Curr Opin Chem Biol,2000,4(5):559−566. doi: 10.1016/S1367-5931(00)00132-0
[16] Li FL,Bao WK. New insights into leaf and fine-root trait relationships:implications of resource acquisition among 23 xerophytic woody species[J]. Ecol Evol,2015,5(22):5344−5351. doi: 10.1002/ece3.1794
[17] Strahm BD,Harrison RB. Nitrate sorption in a variable-charge forest soil of the Pacific northwest[J]. Soil Sci,2006,171(4):313−321. doi: 10.1097/01.ss.0000209355.76407.16
[18] 黄慧彧. 根系修剪对水稻幼苗根系形态特征和氮素吸收的影响[D]. 武汉:华中农业大学,2022:10−21. [19] Crawford NM,Glass ADM. Molecular and physiological aspects of nitrate uptake in plants[J]. Trends Plant Sci,1998,3(10):389−395. doi: 10.1016/S1360-1385(98)01311-9
[20] Kattge J,Bönisch G,Díaz S,Lavorel S,Prentice IC,et al. TRY plant trait database-enhanced coverage and open access[J]. Global Change Biol,2020,26(1):119−188. doi: 10.1111/gcb.14904
[21] Sun ZZ,Liu LL,Ma YC,Yin GD,Zhao C,et al. The effect of nitrogen addition on soil respiration from a nitrogen-limited forest soil[J]. Agric For Meteorol,2014,197:103−110. doi: 10.1016/j.agrformet.2014.06.010
[22] Jampeetong A,Brix H. Effects of NH4+ concentration on growth,morphology and NH4+ uptake kinetics of Salvinia natans[J]. Ecol Eng,2009,35(5):695−702. doi: 10.1016/j.ecoleng.2008.11.006
[23] Aslam M,Travis RL,Huffaker RC. Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings[J]. Plant Physiol,1992,99(3):1124−1133. doi: 10.1104/pp.99.3.1124
[24] 鲜玲. 沉水植物铵同化特征的研究——以穗状狐尾藻和光叶眼子菜为例[D]. 北京:中国科学院大学,2020:52−59. [25] Alboresi A,Gestin C,Leydecker MT,Bedu M,Meyer C,Truong HN. Nitrate,a signal relieving seed dormancy in Arabidopsis[J]. Plant Cell Environ,2005,28(4):500−512. doi: 10.1111/j.1365-3040.2005.01292.x
[26] Krouk G,Lacombe B,Bielach A,Perrine-Walker F,Malinska K,et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants[J]. Dev Cell,2010,18(6):927−937. doi: 10.1016/j.devcel.2010.05.008
[27] Filleur S,Dorbe MF,Cerezo M,Orsel M,Granier F,et al. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake[J]. FEBS Lett,2001,489(2-3):220−224. doi: 10.1016/S0014-5793(01)02096-8
[28] Kiba T,Feria-Bourrellier AB,Lafouge F,Lezhneva L,Boutet-Mercey S,et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants[J]. Plant Cell,2012,24(1):245−258. doi: 10.1105/tpc.111.092221
[29] Nayar S,Loo MGK,Tanner JE,Longmore AR,Jenkins GP. Nitrogen acquisition and resource allocation strategies in temperate seagrass Zostera nigricaulis:uptake,assimilation,and translocation processes[J]. Sci Rep,2018,8(1):17151. doi: 10.1038/s41598-018-35549-3
[30] Ludewig U,Neuhäuser B,Dynowski M. Molecular mechanisms of ammonium transport and accumulation in plants[J]. FEBS Lett,2007,581(12):2301−2308. doi: 10.1016/j.febslet.2007.03.034
[31] Marschner H. Preface to First Edition[M]. Amsterdam:Elsevier,2012:vii.
[32] Yuan LX,Loque D,Kojima S,Rauch S,Ishiyama K,et al. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters[J]. Plant Cell,2007,19(8):2636−2652. doi: 10.1105/tpc.107.052134
[33] Wang M,Shen QR,Xu GH,Guo SW. New insight into the strategy for nitrogen metabolism in plant cells[J]. Int Rev Cell Mol Biol,2014,310:1−37.
[34] Loqué D,Yuan LX,Kojima S,Gojon A,Wirth J,et al. Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots[J]. Plant J,2006,48(4):522−534. doi: 10.1111/j.1365-313X.2006.02887.x
[35] Gazzarrini S,Lejay L,Gojon A,Ninnemann O,Frommer WB,von Wirén N. Three functional transporters for constitutive,diurnally regulated,and starvation-induced uptake of ammonium into Arabidopsis roots[J]. Plant Cell,1999,11(6):937−947.
[36] Couturier J,Montanini B,Martin F,Brun A,Blaudez D,Chalot M. The expanded family of ammonium transporters in the perennial poplar plant[J]. New Phytol,2007,174(1):137−150. doi: 10.1111/j.1469-8137.2007.01992.x
[37] Sonoda Y,Ikeda A,Saiki S,Yamaya T,Yamaguchi J. Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice[J]. Plant Cell Physiol,2004,45(12):1396−1402.
[38] Van den Berg MS,Scheffer M,Coops H,Simons J. The role of characean algae in the management of eutrophic shallow lakes[J]. J Phycol,1998,34(5):750−756. doi: 10.1046/j.1529-8817.1998.340750.x
[39] Jeppesen E,Jensen JP,Søndergaard M,Lauridsen T,Pedersen LJ,Jensen L. Top-down control in freshwater lakes:the role of nutrient state,submerged macrophytes and water depth[J]. Hydrobiologia,1997,342:151−164.
[40] Xiong HF. Uptake kinetics of NH4+,NO3− and H2PO4− by submerged macrophytes Elodea nuttallii and Vallisneria natans (JUSSIEU,1826)[J]. Appl Ecol Environ Res,2019,17(1):1027−1037. doi: 10.15666/aeer/1701_10271037
[41] Olesen A,Jensen SM,Alnoee AB,Baattrup-Pedersen A,Lauridsen TL,et al. Nutrient kinetics in submerged plant beds:a mesocosm study simulating constructed drainage wetlands[J]. Ecol Eng,2018,122:263−270. doi: 10.1016/j.ecoleng.2018.08.012
[42] 陈少毅,许超,姚瑶,黄立章,张云涛. 黑藻和苦草对氨氮、硝态氮和磷吸收动力学研究[J]. 环境科学与技术,2012,35(8):34−36. doi: 10.3969/j.issn.1003-6504.2012.08.008 Chen SY,Xu C,Yao Y,Huang LZ,Zhang YT. Uptake kinetics of ammonia,nitrate and phosphorus by submerged macrophytes Hydrilla verticillata and Vallisneria natans[J]. Environmental Science & Technology,2012,35(8):34−36. doi: 10.3969/j.issn.1003-6504.2012.08.008
[43] Gu SY,Hsieh CT,Gandomi YA,Chang JK,Li J,et al. Microwave growth and tunable photoluminescence of nitrogen-doped graphene and carbon nitride quantum dots[J]. J Mater Chem C,2019,7(18):5468−5476. doi: 10.1039/C9TC00233B
[44] 钟爱文,曹特,张萌,倪乐意,谢平. 光照和黑暗条件下苦草(Vallisneria natans)和穗花狐尾藻(Myriophyllum spicatum)对铵态氮的吸收[J]. 湖泊科学,2013,25(2):289−294. doi: 10.3969/j.issn.1003-5427.2013.02.017 Zhong AW,Cao T,Zhang M,Ni LY,Xie P. Uptake of ammonium by Vallisneria natans and Myriophyllum spicatum under light and dark regimes[J]. Journal of Lake Sciences,2013,25(2):289−294. doi: 10.3969/j.issn.1003-5427.2013.02.017
[45] Wang Q,Zhang ZH,Du R,Wang SP,Duan JC,et al. Richness of plant communities plays a larger role than climate in determining responses of species richness to climate change[J]. J Ecol,2019,107(4):1944−1955. doi: 10.1111/1365-2745.13148
[46] Xian L,Ochieng WA,Muthui SW,Otieno DO,Yu SW,et al. The above-ground part of submerged macrophytes plays an important role in ammonium utilization[J]. Front Plant Sci,2022,13:865578. doi: 10.3389/fpls.2022.865578
[47] Tercé-Laforgue T,Dubois F,Ferrario-Méry S,de Crecenzo MAP,Sangwan R,et al. Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration[J]. Plant Physiol,2004,136(4):4308−4317. doi: 10.1104/pp.104.047548
[48] Gibson SI. Control of plant development and gene expression by sugar signaling[J]. Curr Opin Plant Biol,2005,8(1):93−102. doi: 10.1016/j.pbi.2004.11.003
[49] Sand-Jensen K,Prahl C. Oxygen exchange with the lacunae and across leaves and roots of the submerged vascular macrophyte,Lobelia dortmanna L.[J]. New Phytol,1982,91(1):103−120. doi: 10.1111/j.1469-8137.1982.tb03296.x
[50] Cedergreen N,Madsen TV. Nitrate reductase activity in roots and shoots of aquatic macrophytes[J]. Aquat Bot,2003,76(3):203−212. doi: 10.1016/S0304-3770(03)00050-0
[51] Cao T,Ni LY,Xie P,Xu J,Zhang M. Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability[J]. Freshwater Biol,2011,56(8):1620−1629. doi: 10.1111/j.1365-2427.2011.02601.x
[52] Paola Rosales E,Florencia Iannone M,Daniela Groppa M,Patricia Benavides M. Nitric oxide inhibits nitrate reductase activity in wheat leaves[J]. Plant Physiol Biochem,2011,49(2):124−130. doi: 10.1016/j.plaphy.2010.10.009
[53] Diaz C,Lemaitre T,Christ A,Azzopardi M,Kato Y,et al. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition[J]. Plant Physiol,2008,147(3):1437−1449. doi: 10.1104/pp.108.119040
[54] Masclaux-Daubresse C,Reisdorf-Cren M,Orsel M. Leaf nitrogen remobilisation for plant development and grain filling[J]. Plant Biol,2008,10(s1):23−36. doi: 10.1111/j.1438-8677.2008.00097.x
[55] Linka M,Weber APM. Shuffling ammonia between mitochondria and plastids during photorespiration[J]. Trends Plant Sci,2005,10(10):461−465. doi: 10.1016/j.tplants.2005.08.002
[56] Masclaux-Daubresse C,Reisdorf-Cren M,Pageau K,Lelandais M,Grandjean O,et al. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco[J]. Plant Physiol,2006,140(2):444−456. doi: 10.1104/pp.105.071910
[57] Blanco L,Reddy PM,Silvente S,Bucciarelli B,Khandual S,et al. Molecular cloning,characterization and regulation of two different NADH-glutamate synthase cDNAs in bean nodules[J]. Plant Cell Environ,2008,31(4):454−472. doi: 10.1111/j.1365-3040.2008.01774.x
[58] Turano FJ,Dashner R,Upadhyaya A,Caldwell CR. Purification of mitochondrial glutamate dehydrogenase from dark-grown soybean seedlings[J]. Plant Physiol,1996,112(3):1357−1364. doi: 10.1104/pp.112.3.1357
[59] Fontaine JX,Tercé-Laforgue T,Bouton S,Pageau K,Lea PJ,et al. Further insights into the isoenzyme composition and activity of glutamate dehydrogenase in Arabidopsis thaliana[J]. Plant Signal Behav,2013,8(3):e23329. doi: 10.4161/psb.23329
[60] Miflin BJ,Lea PJ. Pathway of nitrogen assimilation in plants[J]. Phytochemistry,1976,15(6):873−885. doi: 10.1016/S0031-9422(00)84362-9
[61] Yang XY,Wang XF,Wei M,Hikosaka S,Goto E. Response of ammonia assimilation in cucumber seedlings to nitrate stress[J]. J Plant Biol,2010,53(3):173−179. doi: 10.1007/s12374-010-9096-9
[62] Liu Y,von Wirén N. Ammonium as a signal for philological and morphological responses in plants[J]. J Exp Bot,2017,68(10):2581−2592. doi: 10.1093/jxb/erx086
[63] Sato S,Yanagisawa S. Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis[J]. Plant Cell Physiol,2014,55(2):306−319. doi: 10.1093/pcp/pct192
[64] Britto DT,Kronzucker HJ. NH4+ toxicity in higher plants:a critical review[J]. J Plant Physiol,2002,159(6):567−584. doi: 10.1078/0176-1617-0774
[65] Cao T,Xie P,Li ZQ,Ni LY,Zhang M,Xu J. Physiological stress of high NH4+ concentration in water column on the submersed macrophyte Vallisneria natans L.[J]. Bull Environ Contam Toxicol,2009,82(3):296−299. doi: 10.1007/s00128-008-9531-5
[66] Gao JQ,Li LS,Hu ZY,Yue H,Zhang RQ,Xiong ZT. Effect of ammonia stress on nitrogen metabolism of Ceratophyllum demersum[J]. Environ Toxicol Chem,2016,35(1):205−211. doi: 10.1002/etc.3182
[67] Zhou QY,Gao JQ,Zhang RM,Zhang RQ. Ammonia stress on nitrogen metabolism in tolerant aquatic plant-Myriophyllum aquaticum[J]. Ecotoxicol Environ Saf,2017,143:102−110. doi: 10.1016/j.ecoenv.2017.04.016
[68] Xian L,Zhang YZ,Cao Y,Wan T,Gong YB,et al. Glutamate dehydrogenase plays an important role in ammonium detoxification by submerged macrophytes[J]. Sci Total Environ,2020,722:137859. doi: 10.1016/j.scitotenv.2020.137859
[69] Ochieng WA,Xian L,Nasimiyu AT,Muthui SW,Ndirangu LN,et al. Exploring the ammonium detoxification mechanism of young and mature leaves of the macrophyte Potamogeton lucens[J]. Aquat Toxicol,2021,237:105879. doi: 10.1016/j.aquatox.2021.105879
[70] Wang R,Xu SJ,Jiang CC,Sun HS,Feng SG,et al. Transcriptomic sequencing and co-expression network analysis on key genes and pathways regulating nitrogen use efficiency in Myriophyllum aquaticum[J]. Int J Mol Sci,2019,20(7):1587. doi: 10.3390/ijms20071587



 下载:
下载: