Functional analysis of Solanum lycopersicum L. transcription factor SlERF.F4 against gray mold in tomato fruit
-
摘要:
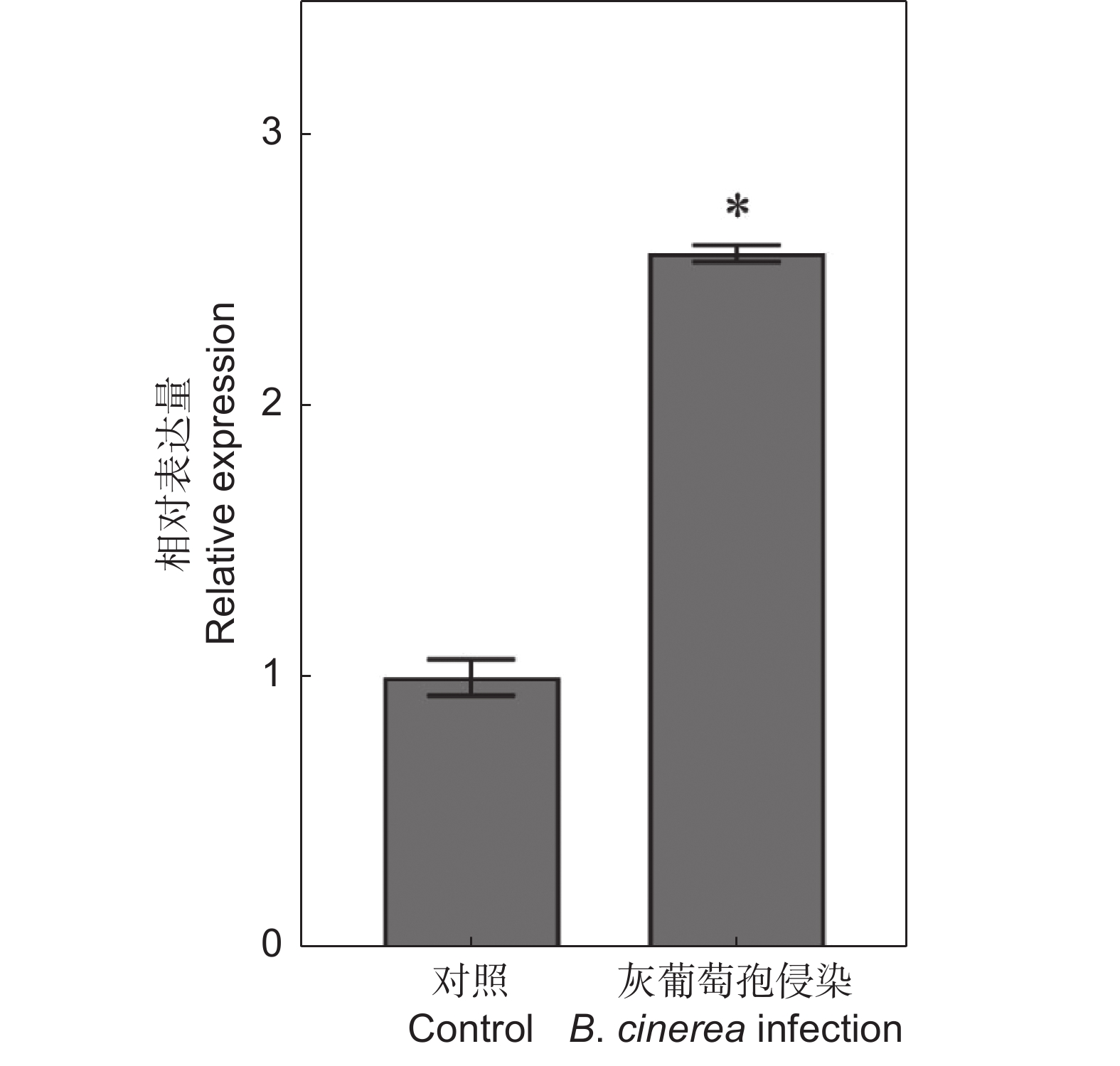
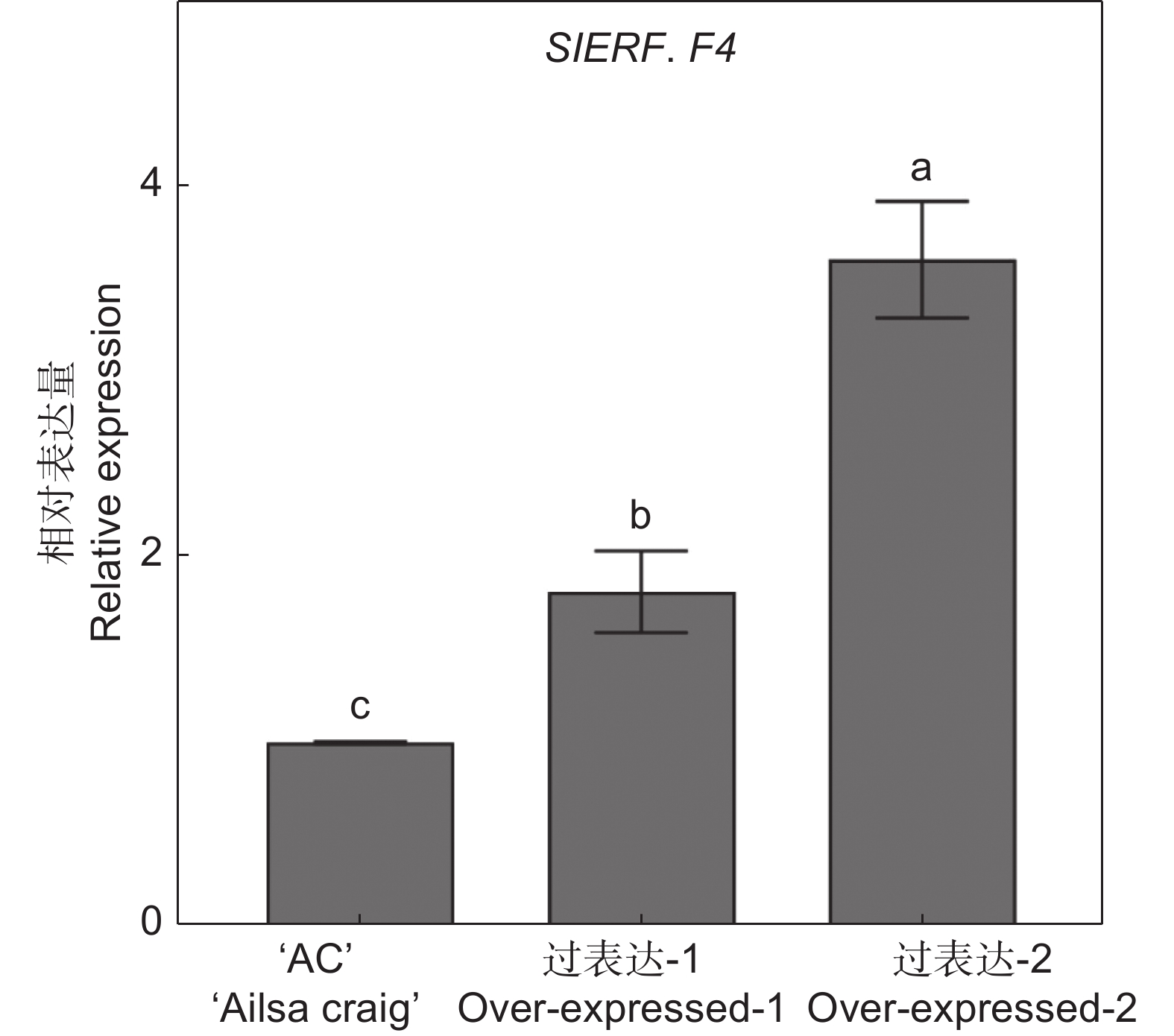
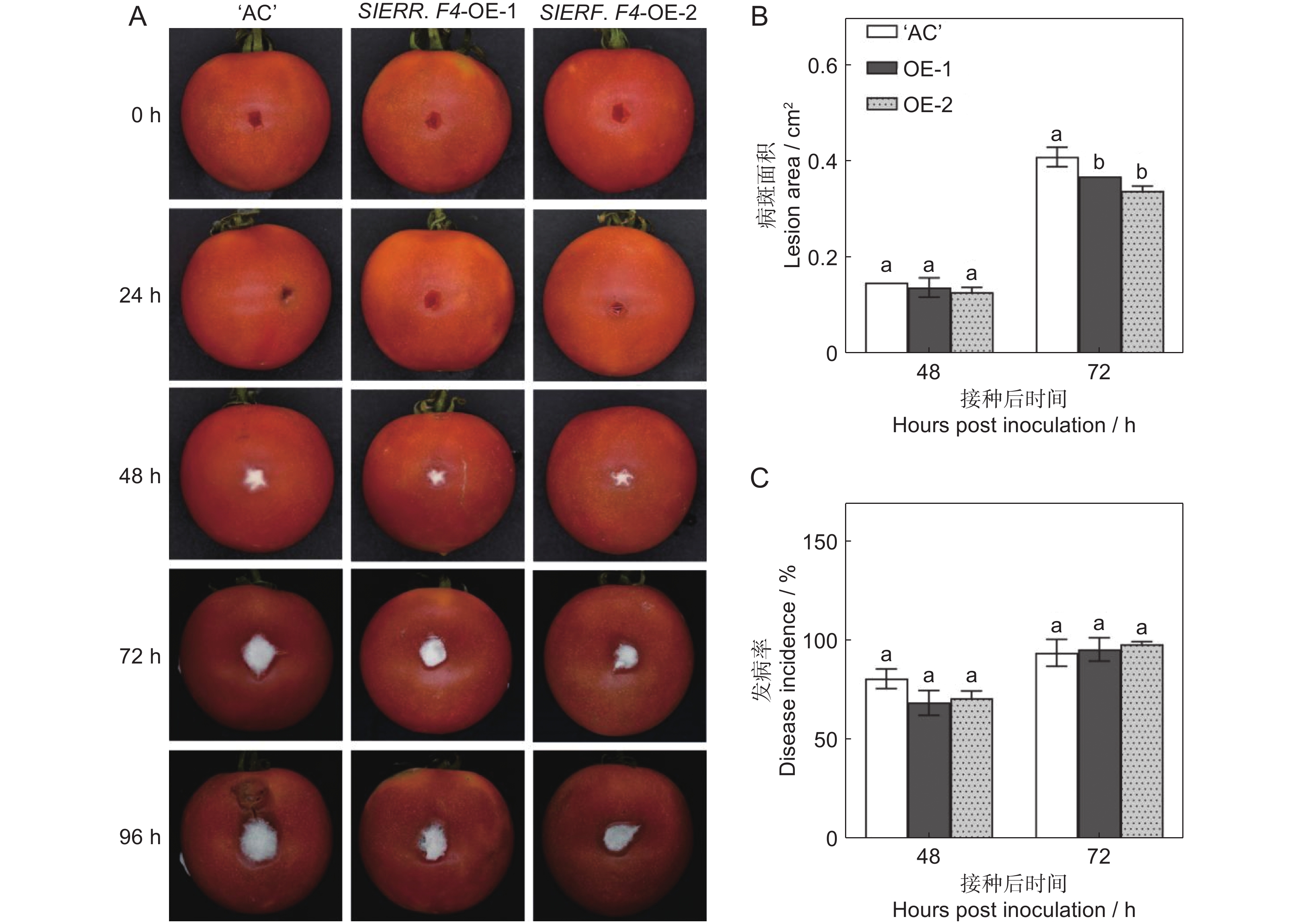
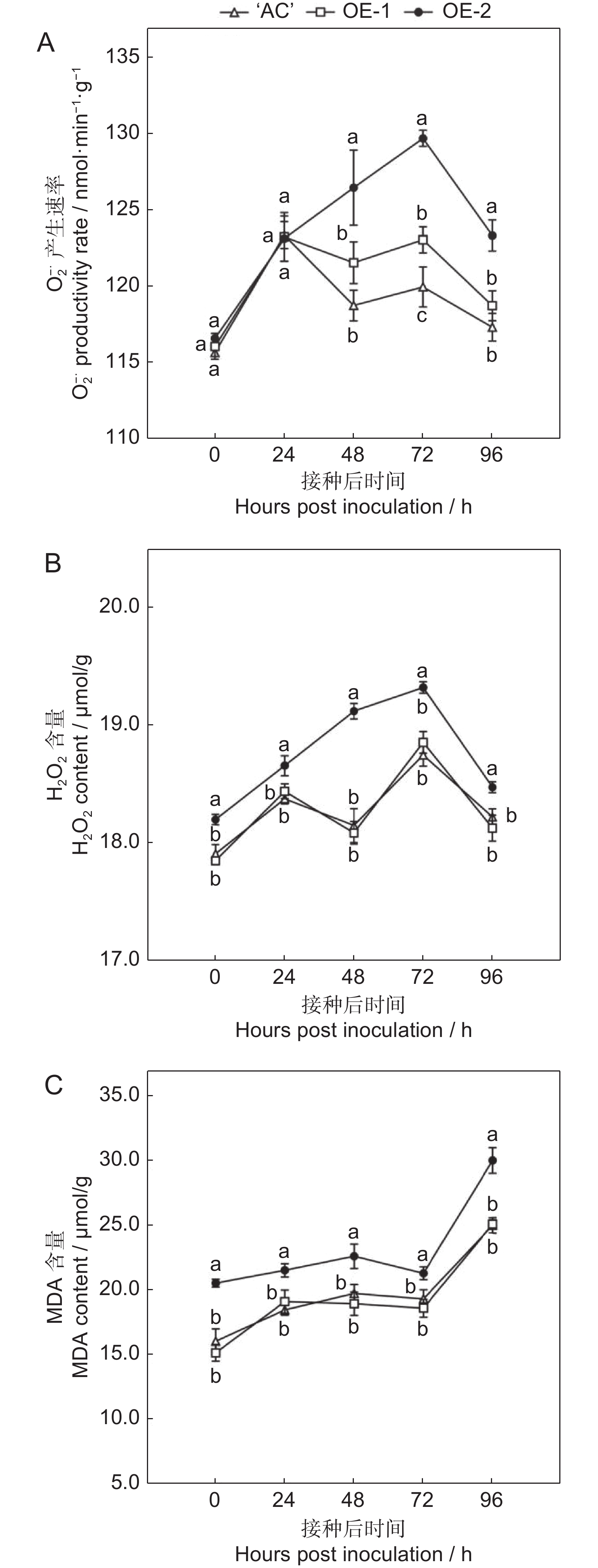
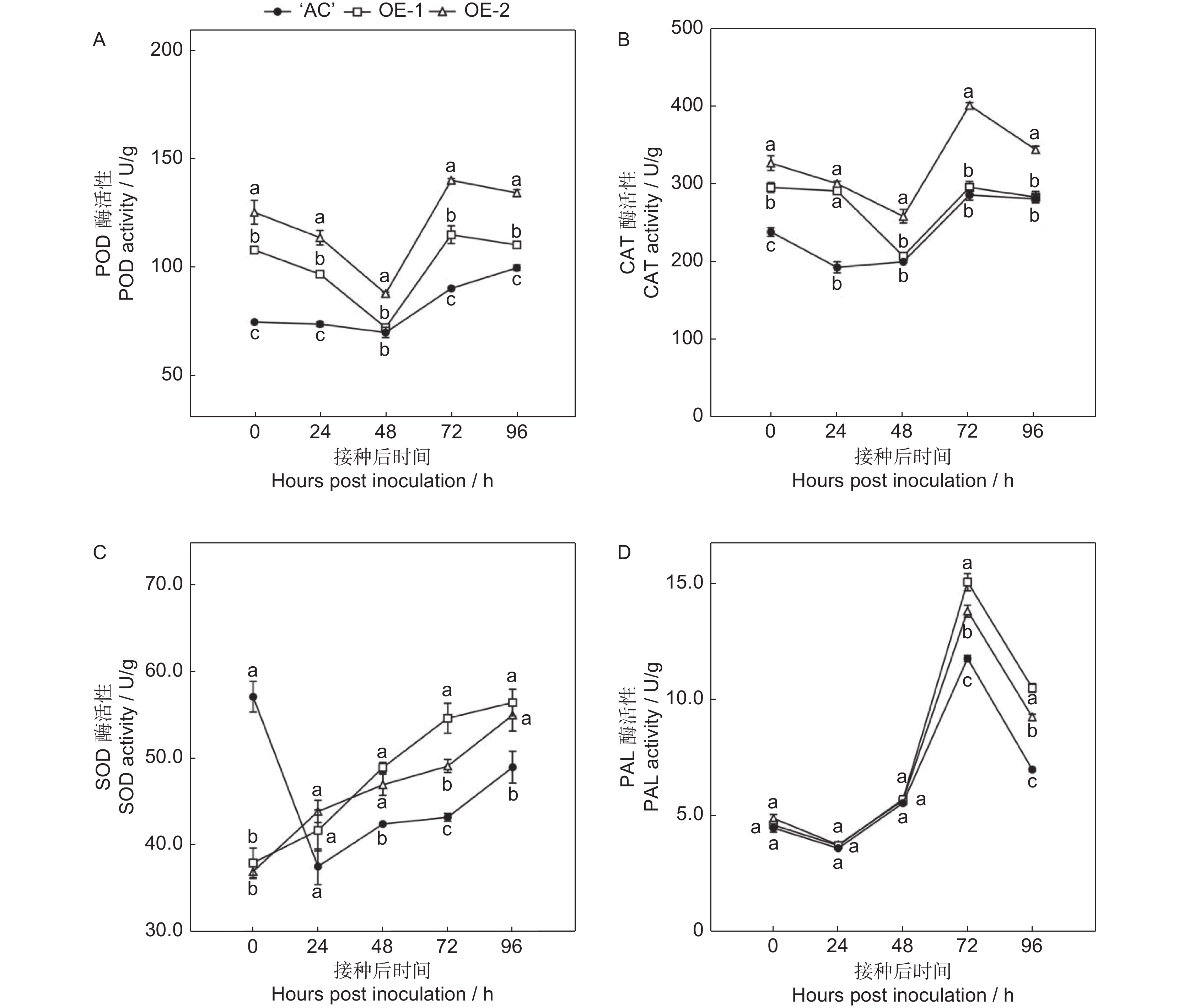
番茄(Solanum lycopersicum L.)是世界上种植最广泛的经济作物之一,具有极大的经济价值,但其极易受到灰葡萄孢(Botrytis cinerea)的影响,造成严重的经济损失。ERF转录因子在植物胁迫响应中发挥关键作用。本研究通过获得番茄SlERF.F4过表达材料,比较其与野生型果实的差异,研究其介导的番茄果实抗灰霉病的功能。结果显示,与野生型番茄品种‘AC’(‘Ailsa Craig’)果实相比,SlERF.F4过表达(SlERF.F4-OE)番茄果实对灰葡萄孢的抗性较强,表现在灰葡萄孢接种后果实病斑扩展速率较慢。生理生化分析结果表明,SlERF.F4-OE果实体内的活性氧(O2−.产生速率、H2O2含量)和MDA含量低于‘AC’果实,同时,抗氧化酶活性(POD、CAT和SOD酶)高于‘AC’果实。此外,SlERF.F4-OE番茄果实体内苯丙氨酸解氨酶(PAL)活性高于‘AC’果实。研究结果说明,SlERF.F4可通过调节番茄果实活性氧稳态及防御酶活性增强果实对灰葡萄孢的抵抗能力。
Abstract:Solanum lycopersicum L. is one of the most widely cultivated cash crops globally, but its susceptibility to gray mold (Botrytis cinerea) causes significant economic losses. ERF transcription factors play a key role in plant stress responses. In this study, SlERF.F4 overexpression lines were generated and compared with wild-type tomato variety ‘AC’ (‘Ailsa Craig’) fruits to investigate the role of SlERF.F4 in mediating resistance to gray mold in tomatoes. Compared with ‘AC’ fruits, SlERF.F4-overexpressed (SlERF.F4-OE) fruits exhibited enhanced resistance to B. cinerea, as indicated by a slower lesion expansion following inoculation. Physiological and biochemical analyses revealed that the production rate of reactive oxygen species (ROS), including O2-. and H2O2 content, as well as malondialdehyde (MDA) levels, were significantly lower in SlERF.F4-OE fruits than in ‘AC’ fruits. Concurrently, antioxidant enzyme activities, including peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD), were significantly elevated in SlERF.F4-OE fruits. In addition, phenylalanine ammonia-lyase (PAL) activity was higher in SlERF.F4-OE fruits than in ‘AC’ fruits. These results suggest that SlERF.F4 enhances resistance to B. cinerea in S. lycopersicum fruits by regulating reactive oxygen homeostasis and defense enzyme activity. This study provides novel insights into the function of SlERF.F4 in fruit disease resistance.
-
Keywords:
- Solanum lycopersicum /
- Botrytis cinerea /
- ERF /
- ROS
-
-
表 1 引物序列
Table 1 Primer sequences used in this study
引物名称
Primer name引物序列(5’→3’)
Primer sequence (5’→3’)SlERF.F4-F AGAGGATCCATGGCTGTGAAAGATA SlERF.F4-R AATTCGAGCTCTTAAACTTCCATAGGT Actin-F CCTCAGCACATTCCAGCAG Actin-R CCACCAAACTTCTCCATCCC qPCR-SlERF.F4-F GAGTCATCCAGCGGAGAAACGG qPCR-SlERF.F4-R GACACCTCCACGTCCACCTTCA Hyg-F CTTGACATTGGGGAGTTTAGCGAGA Hyg-R CCCTTATCTGGGAACTACTCACACA -
[1] Ranjan A,Ichihashi Y,Sinha NR. The tomato genome:implications for plant breeding,genomics and evolution[J]. Genome Biol,2012,13(8):167. doi: 10.1186/gb-2012-13-8-167
[2] Chitwood-Brown J,Vallad GE,Lee TG,Hutton SF. Breeding for resistance to Fusarium wilt of tomato:a review[J]. Genes (Basel),2021,12(11):1673. doi: 10.3390/genes12111673
[3] Escobar-Niño A,Morano Bermejo IM,Carrasco Reinado R,Fernandez-Acero FJ. Deciphering the dynamics of signaling cascades and virulence factors of B. cinerea during tomato cell wall degradation[J]. Microorganisms,2021,9(9):1837. doi: 10.3390/microorganisms9091837
[4] Tian S,Liu BJ,Shen YN,Cao SS,Lai YY,et al. Unraveling the molecular mechanisms of tomatoes' defense against Botrytis cinerea:insights from transcriptome analysis of Micro-Tom and regular tomato varieties[J]. Plants (Basel),2023,12(16):2965. doi: 10.3390/plants12162965
[5] Yang P,Zhao ZZ,Virag A,Becker T,Zhao LJ,et al. Botrytis cinerea in vivo inoculation assays for early-,middle- and late-stage strawberries[J]. Bio-Protoc,2023,13(20):e4859.
[6] Lorenzini M,Azzolini M,Tosi E,Zapparoli G. Postharvest grape infection of Botrytis cinerea and its interactions with other moulds under withering conditions to produce noble-rotten grapes[J]. J Appl Microbiol,2013,114(3):762−770. doi: 10.1111/jam.12075
[7] Kong WW,Chen N,Liu TT,Zhu J,Wang JQ,et al. Large-scale transcriptome analysis of cucumber and Botrytis cinerea during infection[J]. PLoS One,2015,10(11):e0142221. doi: 10.1371/journal.pone.0142221
[8] Swart L,Langenhoven P. First report of Botrytis blight,caused by Botrytis cinerea,on Hibiscus in South Africa[J]. Plant Dis,2000,84(4):487.
[9] Ren HR,Bai MJ,Sun JJ,Liu JY,Ren M,et al. RcMYB84 and RcMYB123 mediate jasmonate-induced defense responses against Botrytis cinerea in rose (Rosa chinensis)[J]. Plant J,2020,103(5):1839−1849.
[10] Mercier A,Carpentier F,Duplaix C,Auger A,Pradier JM,et al. The polyphagous plant pathogenic fungus Botrytis cinerea encompasses host-specialized and generalist populations[J]. Environ Microbiol,2019,21(12):4808−4821. doi: 10.1111/1462-2920.14829
[11] Caseys C,Shi GJ,Soltis N,Gwinner R,Corwin J,et al. Quantitative interactions:the disease outcome of Botrytis cinerea across the plant kingdom[J]. G3 (Bethesda),2021,11(8):jkab175. doi: 10.1093/g3journal/jkab175
[12] Bi K,Liang Y,Mengiste T,Sharon A. Killing softly:a roadmap of Botrytis cinerea pathogenicity[J]. Trends Plant Sci,2023,28(2):211−222. doi: 10.1016/j.tplants.2022.08.024
[13] Nakano T,Suzuki K,Fujimura T,Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice[J]. Plant Physiol,2006,140(2):411−432. doi: 10.1104/pp.105.073783
[14] Feng K,Hou XL,Xing GM,Liu JX,Duan AQ,et al. Advances in AP2/ERF super-family transcription factors in plant[J]. Crit Rev Biotechnol,2020,40(6):750−776. doi: 10.1080/07388551.2020.1768509
[15] Müller M,Munné-Bosch S. Ethylene response factors:a key regulatory hub in hormone and stress signaling[J]. Plant Physiol,2015,169(1):32−41. doi: 10.1104/pp.15.00677
[16] 莫纪波,李大勇,张慧娟,宋凤鸣. ERF转录因子在植物对生物和非生物胁迫反应中的作用[J]. 植物生理学报,2011,47(12):1145−1154. Mo JB,Li DY,Zhang HJ,Song FM. Roles of ERF transcription factors in biotic and abiotic stress response in plants[J]. Plant Physiology Communications,2011,47(12):1145−1154.
[17] Ohme-Takagi M,Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element[J]. Plant Cell,1995,7(2):173−182.
[18] 王雅慧,李彤,黄莹,刘洁霞,王枫,熊爱生. 番茄2个ERF-B1亚族转录因子基因的克隆及其对生物和非生物胁迫响应[J]. 核农学报,2019,33(10):1893−1904. doi: 10.11869/j.issn.100-8551.2019.10.1893 Wang YH,Li T,Huang Y,Liu JX,Wang F,Xiong AS. Cloning and expression analysis under biotic and abiotic stresses of two ERF-B1 group transcription factor genes from Solanum lycopersicum[J]. Journal of Nuclear Agricultural Sciences,2019,33(10):1893−1904. doi: 10.11869/j.issn.100-8551.2019.10.1893
[19] 王宁. 锈菌诱导的小麦TaRIPK1在小麦与条锈菌互作中的功能及作用机理研究[D]. 杨凌:西北农林科技大学,2020:1−147. [20] Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components[J]. Physiol Plant Pathol,1983,23(3):345−357. doi: 10.1016/0048-4059(83)90019-X
[21] Chai HB,Doke N. Activation of the potential of potato leaf tissue to react hypersensitively to Phytophthora infestans by cytospore germination fluid and the enhancement of this potential by calcium ions[J]. Physiol Mol Plant Pathol,1987,30(1):27−37. doi: 10.1016/0885-5765(87)90080-4
[22] Sekizawa Y,Haga M,Hirabayashi E,Takeuchi N,Takino Y. Dynamic behavior of superoxide generation in rice leaf tissue infected with blast fungus and its regulation by some substances[J]. Agric Biol Chem,1987,51(3):763−770.
[23] Ouyang ZG,Liu SX,Huang LH,Hong YB,Li XH,et al. Tomato SlERF. A1,SlERF. B4,SlERF. C3 and SlERF. A3,members of B3 group of ERF family,are required for resistance to Botrytis cinerea[J]. Front Plant Sci,2016,7:1964.
[24] Wang J,Higgins VJ. Nitric oxide modulates H2O2-mediated defenses in the Colletotrichum coccodes–tomato interaction[J]. Physiol Mol Plant Pathol,2005,67(3-5):131−137. doi: 10.1016/j.pmpp.2005.11.002
[25] Wang N,Fan X,He MY,Hu ZY,Tang CL,et al. Transcriptional repression of TaNOX10 by TaWRKY19 compromises ROS generation and enhances wheat susceptibility to stripe rust[J]. Plant Cell,2022,34(5):1784−1803. doi: 10.1093/plcell/koac001
[26] 吴雪霞. 外源一氧化氮对盐胁迫下番茄幼苗生理特性影响的研究[D]. 南京:南京农业大学,2007:1−122. [27] Mittler R,Zandalinas SI,Fichman Y,van Breusegem F. Reactive oxygen species signalling in plant stress responses[J]. Nat Rev Mol Cell Biol,2022,23(10):663−679. doi: 10.1038/s41580-022-00499-2
[28] 俞禄珍,王晨,陆苗. ROS信号分子及其在植物抗病反应中的作用[J]. 陕西林业科技,2015(2):70−72. doi: 10.3969/j.issn.1001-2117.2015.02.020 Yu LZ,Wang C,Lu M. The role of ROS signaling molecules in plant disease resistance response[J]. Shaanxi Forest Science and Technology,2015(2):70−72. doi: 10.3969/j.issn.1001-2117.2015.02.020
[29] Mittler R. Oxidative stress,antioxidants and stress tolerance[J]. Trends Plant Sci,2002,7(9):405−410. doi: 10.1016/S1360-1385(02)02312-9
[30] 王晨芳. 小麦与条锈菌互作过程中活性氧迸发的组织学和细胞化学研究[D]. 杨凌:西北农林科技大学,2008:1−122. [31] Wang LY,Hu JP,Li DS,Reymick OO,Tan XL,Tao N. Isolation and control of Botrytis cinerea in postharvest green pepper fruit[J]. Sci Hortic,2022,302:111159. doi: 10.1016/j.scienta.2022.111159
[32] Tenhaken R,Levine A,Brisson LF,Dixon RA,Lamb C. Function of the oxidative burst in hypersensitive disease resistance[J]. Proc Natl Acad Sci USA,1995,92(10):4158−4163. doi: 10.1073/pnas.92.10.4158
[33] 王启方,王晓云,李浩森,杨晓玉,张锐敏,等. 芳樟醇对灰葡萄孢生长的影响及对番茄灰霉病的防控效果[J]. 应用生态学报,2023,34(1):213−220. Wang QF,Wang XY,Li HS,Yang XY,Zhang RM,et al. Effects of linalool on Botrytis cinerea growth and control of tomato gray mold[J]. Chinese Journal of Applied Ecology,2023,34(1):213−220.
[34] Li S,Wu P,Yu XF,Cao JP,Chen X,et al. Contrasting roles of ethylene response factors in pathogen response and ripening in fleshy fruit[J]. Cells,2022,11(16):2484. doi: 10.3390/cells11162484
[35] Zhang HJ,Li KL,Zhang XJ,Dong CH,Ji HP,et al. Effects of ozone treatment on the antioxidant capacity of postharvest strawberry[J]. RSC Adv,2020,10(63):38142−38157. doi: 10.1039/D0RA06448C
[36] 曹建康,姜微波,赵玉梅. 果蔬采后生理生化实验指导[M]. 北京:中国轻工业出版社,2007:1−176. [37] Zhao HD,Liu BD,Zhang WL,Cao JK,Jiang WB. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage[J]. Postharvest Biol Technol,2019,147:113−122. doi: 10.1016/j.postharvbio.2018.09.013
[38] Heath RL,Packer L. Photoperoxidation in isolated chloroplasts:Ⅰ. Kinetics and stoichiometry of fatty acid peroxidation[J]. Arch Biochem Biophys,1968,125(1):189−198. doi: 10.1016/0003-9861(68)90654-1
[39] 郑鄢燕. SlMPK1/2/3在外源NO诱导的采后番茄果实抗病途径中的作用[D]. 北京:中国农业大学,2015:1−136. [40] Trevisan MTS,Scheffer JJC,Verpoorte R. Effect of elicitation on the peroxidase activity in some cell suspension cultures of hop,t Humulus lupulus[J]. Plant Cell Tiss Org Culture,1997,48(2):121−126. doi: 10.1023/A:1005824927070
[41] Patra HK,Kar M,Mishra D. Catalase activity in leaves and cotyledons during plant development and senescence[J]. Biochem Physiol Pflanz,1978,172(4):385−390. doi: 10.1016/S0015-3796(17)30412-2
[42] Havir EA,McHale NA. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves[J]. Plant Physiol,1987,84(2):450−455. doi: 10.1104/pp.84.2.450
[43] Stewart RR,Bewley J D. Lipid peroxidation associated with accelerated aging of soybean axes[J]. Plant Physiol,1980,65(2):245−248. doi: 10.1104/pp.65.2.245
[44] Toivonen PMA,Sweeney M. Differences in chlorophyll loss at 13 °C for two Broccoli (Brassica oleracea L.) cultivars associated with antioxidant enzyme activities[J]. J Agric Food Chem,1998,46(1):20−24. doi: 10.1021/jf970490n
[45] Assis JS,Maldonado R,Muñoz T,Escribano MI,Merodio C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit[J]. Postharvest Biol Technol,2001,23(1):33−39. doi: 10.1016/S0925-5214(01)00100-4
[46] Jin HB,Wang DW,Wang XF. A novel module regulating ROS in NLR-mediated immunity[J]. Trends Plant Sci,2023,28(5):512−514. doi: 10.1016/j.tplants.2023.02.002
[47] 吴顺,萧浪涛. 植物体内活性氧代谢及其信号传导[J]. 湖南农业大学学报(自然科学版),2003,29(5):450−456. doi: 10.3321/j.issn:1007-1032.2003.05.024 Wu S,Xiao LT. Metabolism and signaling conduction of the reactive oxygen species in plant[J]. Journal of Hunan Agricultural University (Natural Sciences),2003,29(5):450−456. doi: 10.3321/j.issn:1007-1032.2003.05.024
[48] Zheng YY,Yang Y,Liu C,Chen L,Sheng JP,Shen L. Inhibition of SlMPK1,SlMPK2,and SlMPK3 disrupts defense signaling pathways and enhances tomato fruit susceptibility to Botrytis cinerea[J]. J Agric Food Chem,2015,63(22):5509−5517. doi: 10.1021/acs.jafc.5b00437
[49] Su YC,Guo JL,Ling H,Chen SS,Wang SS,et al. Isolation of a novel peroxisomal catalase gene from sugarcane,which is responsive to biotic and abiotic stresses[J]. PLoS One,2014,9(1):e84426. doi: 10.1371/journal.pone.0084426
[50] Dong NQ,Lin HX. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions[J]. J Integr Plant Biol,2021,63(1):180−209. doi: 10.1111/jipb.13054
[51] Rohde A,Morreel K,Ralph J,Goeminne G,Hostyn V,et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid,amino acid,and carbohydrate metabolism[J]. Plant Cell,2004,16(10):2749−2771. doi: 10.1105/tpc.104.023705
[52] Bhuiyan NH,Selvaraj G,Wei YD,King J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion[J]. J Exp Bot,2009,60(2):509−521. doi: 10.1093/jxb/ern290
[53] Shu P,Zhang SJ,Li YJ,Wang XY,Yao L,et al. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways[J]. Plant Physiol Biochem,2021,166:1−9. doi: 10.1016/j.plaphy.2021.05.021



 下载:
下载: