Mechanism underlying silver nanoparticle induction of root cell damage and negative gravitropism in main roots of Arabidopsis thaliana (L.) Heynh.
-
摘要:
本研究以拟南芥(Arabidopsis thaliana (L.) Heynh.)为材料,分析了纳米银(AgNPs)对根系生长、根细胞损伤和主根负向重力性的影响。结果显示:(1)30 mg/L AgNPs处理后,拟南芥根系中积累了大量Ag元素,显著高于0.12 mg/L Ag+(30 mg/L AgNPs中释放的Ag+浓度)处理组。(2)30 mg/L AgNPs显著抑制根毛数量和根毛长度,并抑制根毛细胞发育相关基因AtRHS12、AtRHS14、AtCOW1和AtMRH12的表达。(3)30 mg/L的AgNPs或Ag+均能显著诱导根系胼胝质的形成,并损伤根细胞,而0.12 mg/L Ag+处理后根系损伤不明显。(4)30 mg/L AgNPs导致拟南芥主根呈螺旋状生长,而30 mg/L及0.12 mg/L的Ag+则引发主根向左偏移。转录组测序及RT-qPCR分析结果表明,30 mg/L的 AgNPs或Ag+均显著下调乙烯调控相关基因的表达,并上调乙烯合成关键酶基因AtACS7的表达。研究结果表明,30 mg/L AgNPs抑制拟南芥根毛生长,诱导根细胞损伤,并可能通过乙烯信号通路调控根的弯曲生长,其毒性效应可能来源于纳米银本身的颗粒效应。
Abstract:This study explored the effects of silver nanoparticles (AgNPs) on root growth, cellular integrity, and negative gravitropism in Arabidopsis thaliana (L.) Heynh.. Results revealed that: (1) Treatment with 30 mg/L AgNPs led to a significant accumulation of Ag in roots, surpassing the levels observed in roots treated with 0.12 mg/L Ag+ (equivalent to the Ag+ released from 30 mg/L AgNPs). (2) Exposure to 30 mg/L AgNPs markedly inhibited the number and length of root hairs. Transcriptome sequencing and RT-qPCR analyses indicated that 30 mg/L AgNPs suppressed the expression of key genes associated with root hair cell development, including AtRHS12, AtRHS14, AtCOW1, and AtMRH12. (3) Both 30 mg/L AgNPs and 30 mg/L Ag+ induced root callus formation and caused significant root cell damage in A. thaliana. However, no significant root damage was observed in plants treated with 0.12 mg/L Ag+, suggesting that the Ag+ released from AgNPs was insufficient to cause cellular damage. (4) Exposure to 30 mg/L AgNPs induced a spiral growth pattern in the main root, contrasting with the leftward growth induced by 30 mg/L and 0.12 mg/L Ag+. Transcriptome sequencing and RT-qPCR analyses revealed that both 30 mg/L AgNPs and 30 mg/L Ag+ significantly down-regulated ethylene-regulated genes such as AtERS1, AtETR2, AtERF1, AtERF11, and AtEBP, while up-regulating the key ethylene synthesis gene AtACS7. These findings suggest that AgNPs and Ag+ may influence negative gravitropism in A. thaliana roots by modulating ethylene signaling pathways and ethylene biosynthesis. In conclusion, 30 mg/L AgNPs inhibit root hair growth, induce root cell damage, and influence gravitropic bending in A. thaliana roots through the ethylene signaling pathway. The observed toxic effects are likely attributable to the intrinsic properties of the nanoparticles.
-
Keywords:
- Silver nanoparticles /
- Root negative gravitropism /
- Ethylene /
- Arabidopsis thaliana
-
纳米银颗粒(AgNPs)因其卓越的抗菌性能已广泛应用于农业、医疗、纺织和食品包装产品中[1, 2]。AgNPs产品的广泛使用让其不可避免地释放到环境中,对生态系统和人类健康产生影响[3, 4]。研究表明,环境中的AgNPs主要分布在土壤和水体中,淡水生态系统中,地表水和底泥中的AgNPs浓度可能接近μg/L级别[5]。每年农用土壤中Ag的输入量约为0.001 2~9.68 μg/kg[6],预计到2050年,农业土壤中的AgNPs最高浓度可能达到10 μg/kg[5]。随着排放到环境中的量日益增多,AgNPs对植物生长发育的影响也开始受到研究者的关注[7]。据报道,AgNPs对生菜(Lactuca sativa L.)[8]、紫萍(Spirodela polyrhiza (L.) Schleid.)[9]、拟南芥(Arabidopsis thaliana (L.) Heynh.)[10]具有毒性效应,并干扰许多生物学过程,包括种子萌发、生物量积累、根系伸长、氧化还原反应和光合作用等[11, 12]。在细胞水平上的研究发现,AgNPs能破坏植物细胞壁,并进入细胞内,在细胞壁和胞间连丝中沉积或聚集[12, 13],这可能会导致细胞机械损伤[14],从而对植物细胞产生毒性。在遗传水平上的研究发现,AgNPs处理会导致拟南芥基因转录水平发生改变,从而延迟植物开花[15]。AgNPs处理洋葱(Allium cepa L.)根尖会导致细胞核内染色体畸变(染色体断裂、形成染色质桥及微核等)以及分裂指数下降,最终抑制细胞分裂[16,17]。
植物吸收是AgNPs进入食物链的主要途径,当根系从土壤中吸收水分和营养时也会同时吸收污染物。AgNPs可以通过根表皮被根冠吸收,并积累在柱细胞中[13, 18],植物根系中Ag的积累量明显高于叶片[19]。研究表明, AgNPs浓度较低时促进根系生长,而浓度较高时则抑制根系生长[20, 21]。此外,暴露于AgNPs的植物根系通常表现出重量减轻和形态改变[22],2.5 mg/L AgNPs处理拟南芥后,其根重降低63%[15],100 mg/L AgNPs则明显破坏根的内部结构[23]。但目前,有关AgNPs影响根系生长发育的报道主要集中在形态水平上,从基因水平探讨其对根系影响的研究仍然缺乏。根系向重力性是植物感知重力并相应调整根系生长方向的重要机制,确保了植物在土壤中获得最佳的营养吸收和定位[24, 25]。乙烯和生长素等植物激素通过影响根系细胞的伸长、分裂和定向生长,在根向重力性中发挥重要作用[26]。适量的乙烯和脱落酸可以促进根系生长,浓度过高则抑制根系生长[27]。也有研究发现,根向重力性取决于初生根尖中的生长素分布[28],细胞分裂素通过调控生长素外流载体蛋白PIN3和PIN7的表达,从而影响根负向重力性[29]。尽管已有大量研究表明AgNPs影响植物根系的生长发育[30, 31],但其如何通过植物激素调控根向重力性尚不明确。
本研究以模式植物拟南芥为实验材料,系统地探究AgNPs对其根系Ag的积累、根负向重力性、根毛生长、根细胞损伤和转录组水平的影响。考虑到AgNPs颗粒会释放出Ag+[8, 32],本研究也设置了Ag+处理组作为对照。研究结果旨在揭示AgNPs对植物根系生长及根负向重力性的影响及其潜在机制,以期为AgNPs的生物安全性评价提供科学依据。
1. 材料与方法
1.1 实验材料
模式植物拟南芥Col-0种子由海南大学热带生物资源可持续利用中心提供,加入硅胶于常温(25 ℃)干燥处存储。
1.2 实验方法
1.2.1 植物培养与实验设计
本研究使用的AgNPs(粒径为(32.1±1.6) nm,zeta电位为(−30.6±0.9) mV)采用多羟基化合物法合成[10]。拟南芥种子用0.9% NaClO溶液消毒15 min,无菌水冲洗3次后,4 ℃低温暗处理72 h,以打破休眠。将种子放置在含有不同浓度处理液的1/2 MS固体培养基上。4个处理分别为CK、30 mg/L AgNPs、30 mg/L Ag+、0.12 mg/L Ag+(30 mg/L AgNPs中释放的Ag+浓度),每个处理3个生物学重复。将平板放置到光照培养箱中进行垂直培养,温度为(22±1)℃,光周期为16 h光照/8 h黑暗,光照强度为125 μmol·m−2· s−1。
1.2.2 根组织中银含量的测定
取AgNPs和Ag+处理6 d后的拟南芥幼苗约0.2 g,用10 mmol/L EDTA-Na2溶液冲洗3次,再用超纯水清洗3次,去除表面残留的银离子。幼苗根和地上部分别于70 ℃烘箱中干燥24 h。干燥后的样品用5 mL 的HNO3在120 ℃下酸解2 h,使用ICP-MS(XSeries 2,Thermo-Fisher,德国)测定银含量。
1.2.3 主根长度、根毛数量和长度测定
将处理3、6 d的拟南芥幼苗取出,清理干净,将培养皿置于全自动体视荧光显微镜(LEICA,M205 FA)下,观察并记录根尖部位0~2 mm处的根毛生长情况,每次拍照时选用相同的放大倍数,每个处理3个重复,每个重复10~15株幼苗。再用相机(Nikon,D7100)拍摄根部照片,使用Image J(v1.52)软件对主根长度、根毛长度和数量进行统计分析。
1.2.4 拟南芥根尖细胞活性鉴定
取处理3 d的拟南芥幼苗,对其根部分别用10 μg/mL的碘化丙啶(PI)和1%(W/V)的苯胺蓝染液进行避光染色。根据具体情况调整染液体积,浸没样品即可,染色20~30 min后,用0.2 mmol/L的磷酸缓冲液(PBS,pH值为7.0)清洗干净,制片后在激光共聚焦显微镜(LEICA,TCS SP8)下观察。PI染色后观察的激发光波长为561 nm,发射光为600~670 nm,呈红色荧光[33];苯胺蓝染色后的激发光波长为405 nm,发射光600~670 nm,呈蓝绿色荧光[34]。
1.2.5 根系转录组测序分析
分别取CK、30 mg/L AgNPs、30 mg/L Ag+处理6 d后的拟南芥幼苗根部约0.1 g,装入预冷的1.5 mL EP管中,放入液氮中速冻。使用Trizol法提取总RNA,超微量分光光度计检测RNA的质量。使用VAHTSTM Total RNA-seq(H/M/R)进行cDNA文库的构建,由上海烈冰生物科技公司进行转录组测序。获得的测序数据已上传至NCBI数据库(GEO,GSE240491)。使用HISAT2软件,对照拟南芥参考基因组(ARAPORT11)进行测序数据分析,基于国际通用的差异筛选算法(EBSeq)对差异基因进行筛选,筛选标准为:|Log2FC|≥1,FDR<0.05。将所有差异基因进行层级聚类分析(R 4.1.2软件,pheatmap 包),并进行GO功能分析(Cytoscape,BINGO)。
1.2.6 实时荧光定量PCR(RT-qPCR)
为了验证转录组测序结果,选择17个与乙烯调控、根生长发育有关的差异基因进行RT-qPCR分析。使用PrimeScript RT试剂盒(Takara)合成cDNA,利用SYBR® Premix Ex TaqTM Ⅱ反应体系进行荧光定量PCR(ABI7500 Fast Real-Time PCR System)。反应程序为两步法(预变性95 ℃,30 s;95 ℃,5 s;65 ℃,30 s,40个循环)。使用Premier 5软件设计基因特异引物,以18S rRNA基因为看家基因,采用2-ΔΔCt方法计算相对基因表达量。引物见附表1
1 )。1.2.7 统计分析
所有数据计算平均值和标准差后,利用Origin 2022软件作图,使用SPSS 16.0软件对不同处理间的差异进行单因素方差分析(One-way ANOVA),采用Duncan检测法进行多重比较。
2. 结果与分析
2.1 拟南芥叶片和根系对银的吸收
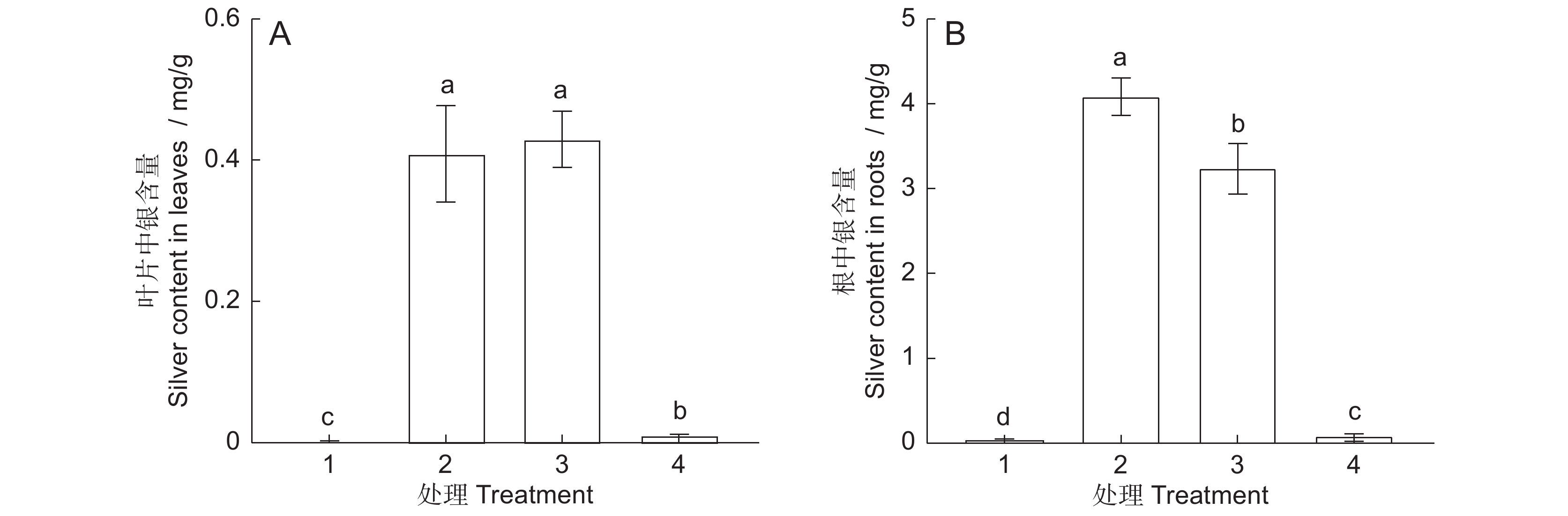
与对照组相比, AgNPs或 Ag+处理6 d后,拟南芥幼苗叶片和根中积累了大量的银,且根部的积累量显著高于叶片(图1)。30 mg/L AgNPs与30 mg/L Ag+处理组叶片中的银含量差异不显著。但30 mg/L AgNPs处理后,根系累积的银浓度为4.1 mg/g,显著高于30 mg/L Ag+(3.2 mg/g)和0.12 mg/L Ag+处理组(0.08 mg/g)。
![]() 图 1 AgNPs或Ag + 处理6 d后叶片和根中银含量1:CK;2:30 mg/L AgNPs;3:30 mg/L Ag+;4:0.12 mg/L Ag+。不同小写字母表示不同处理间差异显著(P<0.05),下同。Figure 1. Silver content in Arabidopsis thaliana leaves and roots after six days of exposure to AgNPs or Ag+1: CK; 2: 30 mg/L AgNPs; 3: 30 mg/L Ag+; 4: 0.12 mg/L Ag+. Different lowercase letters indicate significant differences at P<0.05 level between different treatments, same below.
图 1 AgNPs或Ag + 处理6 d后叶片和根中银含量1:CK;2:30 mg/L AgNPs;3:30 mg/L Ag+;4:0.12 mg/L Ag+。不同小写字母表示不同处理间差异显著(P<0.05),下同。Figure 1. Silver content in Arabidopsis thaliana leaves and roots after six days of exposure to AgNPs or Ag+1: CK; 2: 30 mg/L AgNPs; 3: 30 mg/L Ag+; 4: 0.12 mg/L Ag+. Different lowercase letters indicate significant differences at P<0.05 level between different treatments, same below.2.2 AgNPs对拟南芥根负向重力性的影响
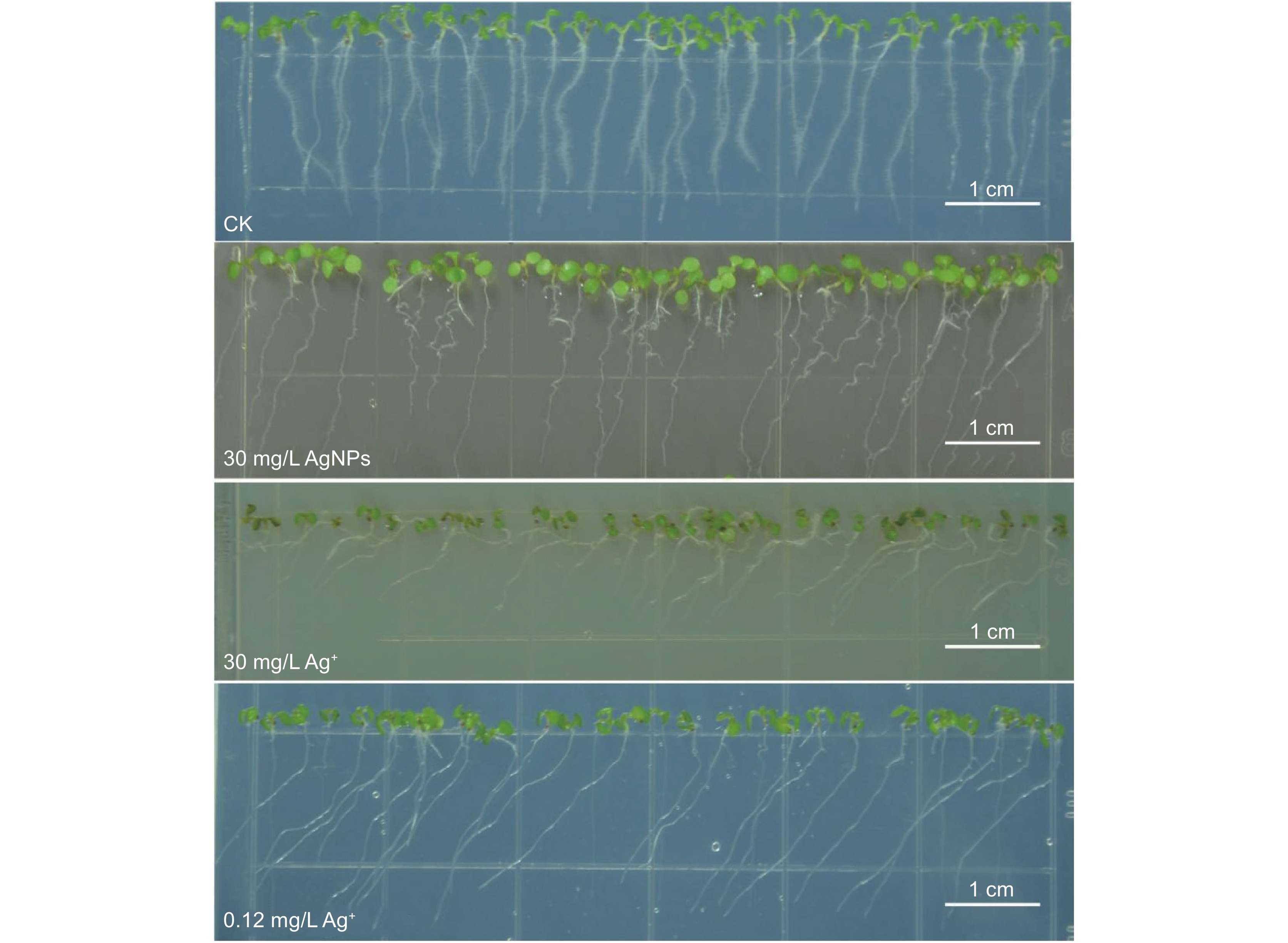
AgNPs和Ag+处理6 d后,拟南芥主根的向重力性明显受损(图2)。30 mg/L AgNPs处理后,拟南芥主根表现出弯曲生长;而30 mg/L Ag+处理后主根则发生明显的向左偏移,偏转角度接近90°;0.12 mg/L Ag+处理后,主根也向左偏移,但偏转角度小于30 mg/L Ag+处理组。
2.3 AgNPs对拟南芥根生长的影响
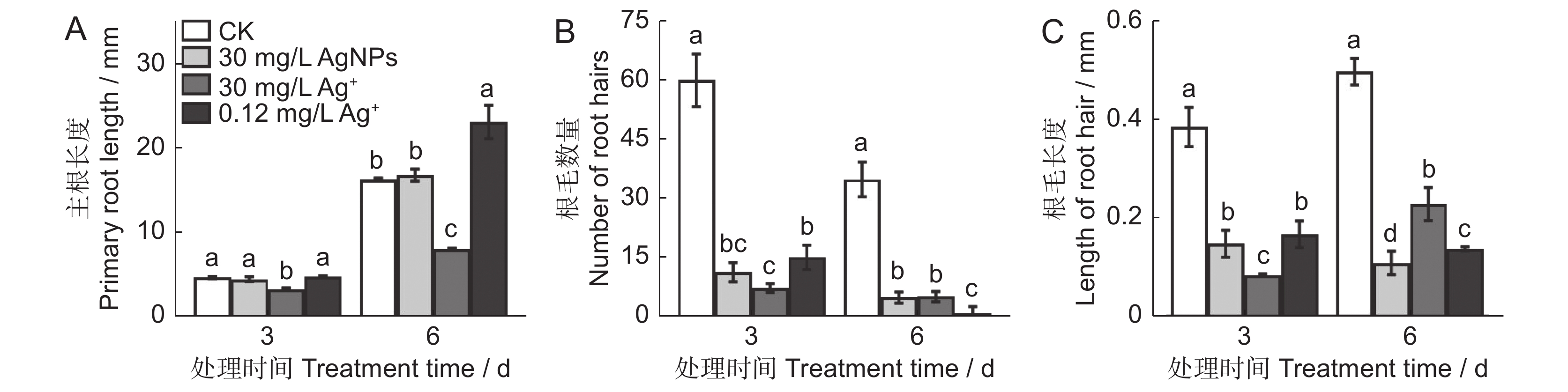
AgNPs和Ag+处理对拟南芥根系生长的影响不同(图3),30 mg/L AgNPs处理6 d后主根的长度与对照组相比无明显差异,30 mg/L Ag+处理6 d后主根的长度被显著抑制,但是0.12 mg/L Ag+处理6 d后显著促进拟南芥主根长度(图3:A)。此外,AgNPs和Ag+处理后拟南芥根尖区域均表现出“无毛”的表型(附图1
2 )),表明根毛受损,根尖失去发育出根毛的能力。与对照相比,AgNPs和Ag+处理3 d和6 d后根毛的数量和长度显著减少(图3:B、C);0.12 mg/L Ag+处理3 d对根毛数量的抑制作用弱于30 mg/L AgNPs和30 mg/L Ag+,但随着处理时间的延长,0.12 mg/L Ag+对根毛数量的抑制作用增强且显著高于30 mg/L AgNPs和30 mg/L Ag+(图3:B、C)。2.4 AgNPs对拟南芥根尖细胞活性的影响
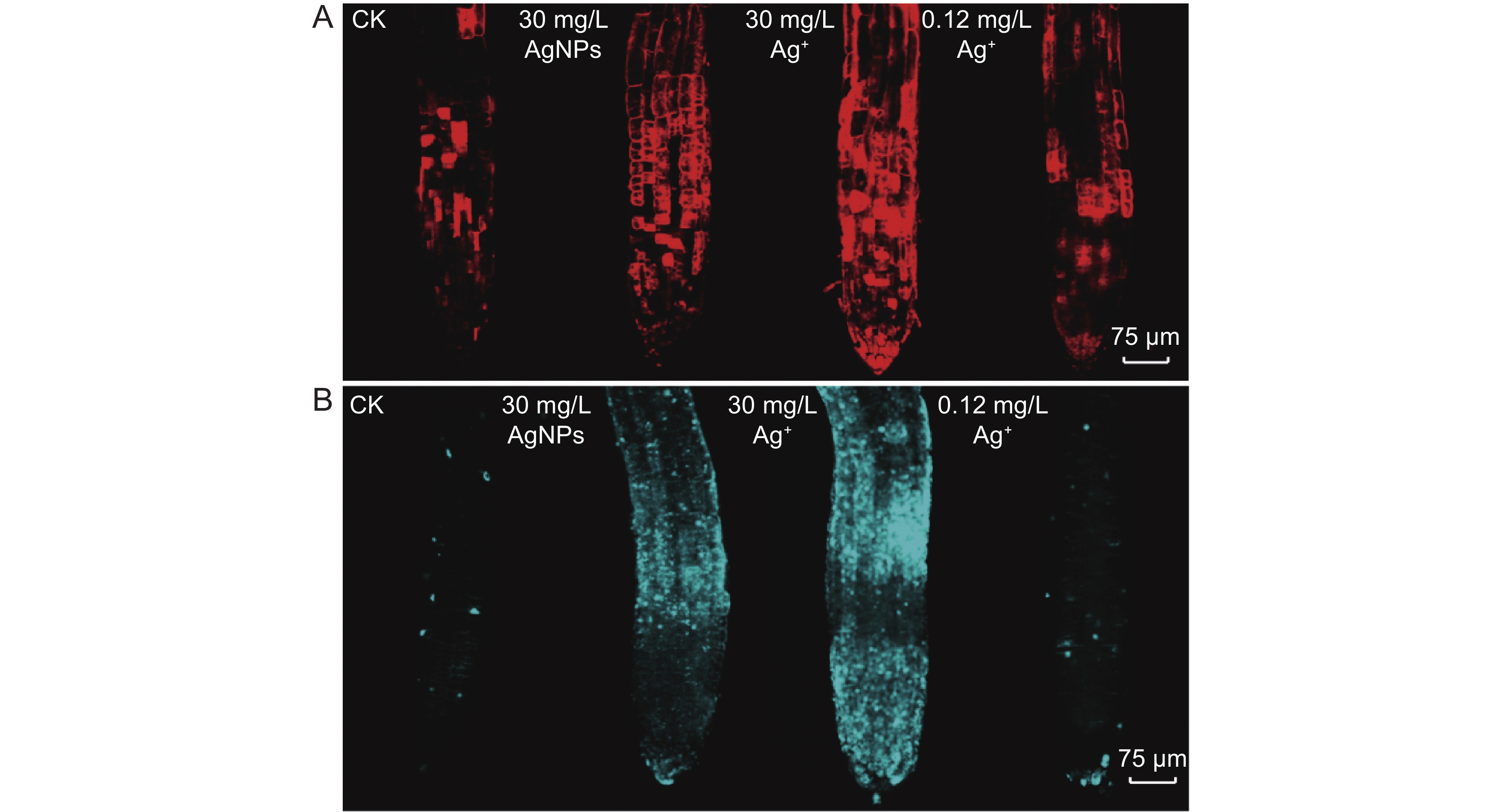
AgNPs和Ag+均可导致拟南芥根细胞损伤(图4)。PI染色结果表明,30 mg/L AgNPs或30 mg/L Ag+处理后,拟南芥根尖均呈红色荧光,且荧光强度显著高于对照组,表明根尖细胞受到明显损伤(图4:A)。苯胺蓝染色结果显示,30 mg/L AgNPs或30 mg/L Ag+处理后,拟南芥根尖均呈蓝色荧光,且荧光强度高于对照组,表明根尖区域细胞壁受损,且有胼胝体沉积(图4:B)。此外,30 mg/L AgNPs处理组的根尖荧光强度明显高于0.12 mg/L Ag+组,而30 mg/L Ag+处理组的荧光强度高于30 mg/L AgNPs。
2.5 AgNPs对拟南芥根系转录组水平的影响
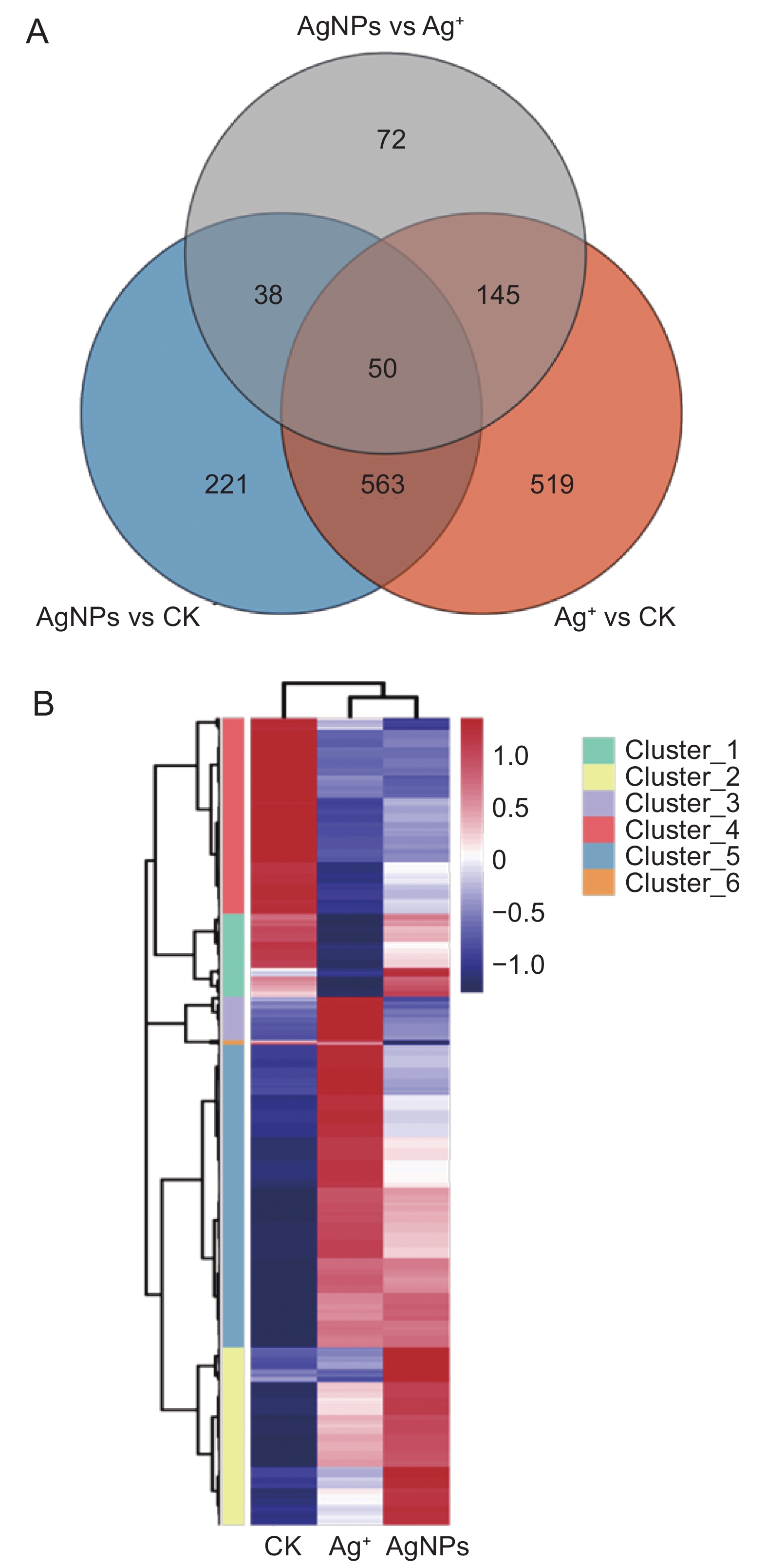
与对照组相比,30 mg/L AgNPs和30 mg/L Ag+分别引起了872和1 277个基因的差异表达(图5:A),其中AgNPs处理组有658个基因上调,214个基因下调;Ag+处理组有794个基因上调,483个基因下调。AgNPs处理组有259个基因特异表达,Ag+引起664个基因发生特异表达,共有613个基因在二者中都发生差异表达。计算所有处理的差异基因,共计1 608个,对这些差异基因进行分层聚类,发现它们分为6个模块(图5:B)。对每个模块进行GO分析,筛选与乙烯调控相关的Item,这些差异基因与乙烯激活信号通路的调控、乙烯激活信号通路的负调控以及与乙烯结合调控等过程相关(附图2
3 ))。聚类分析模块Cluster_1 中的差异基因主要集中在根表皮细胞分化、细胞壁组织、生物合成和根毛细胞分化(附图33 )),Cluster_2 中的大多数差异基因富集在氧化还原过程中,参与防御反应和应激反应的差异基因分别富集在Cluster_3、Cluster_4和Cluster_5(附图43 ))。2.6 AgNPs在转录组水平对拟南芥根毛和乙烯反应的影响
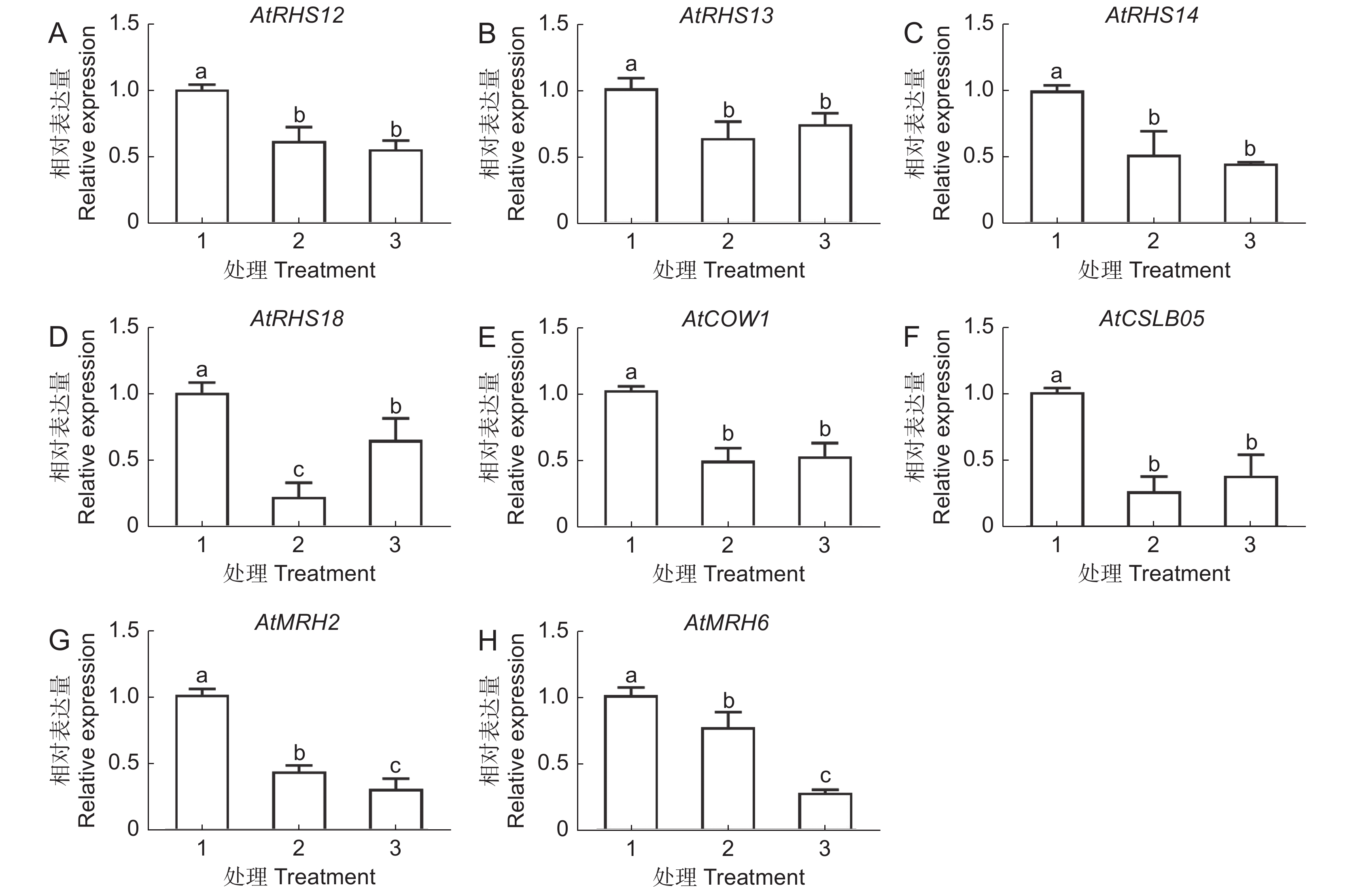
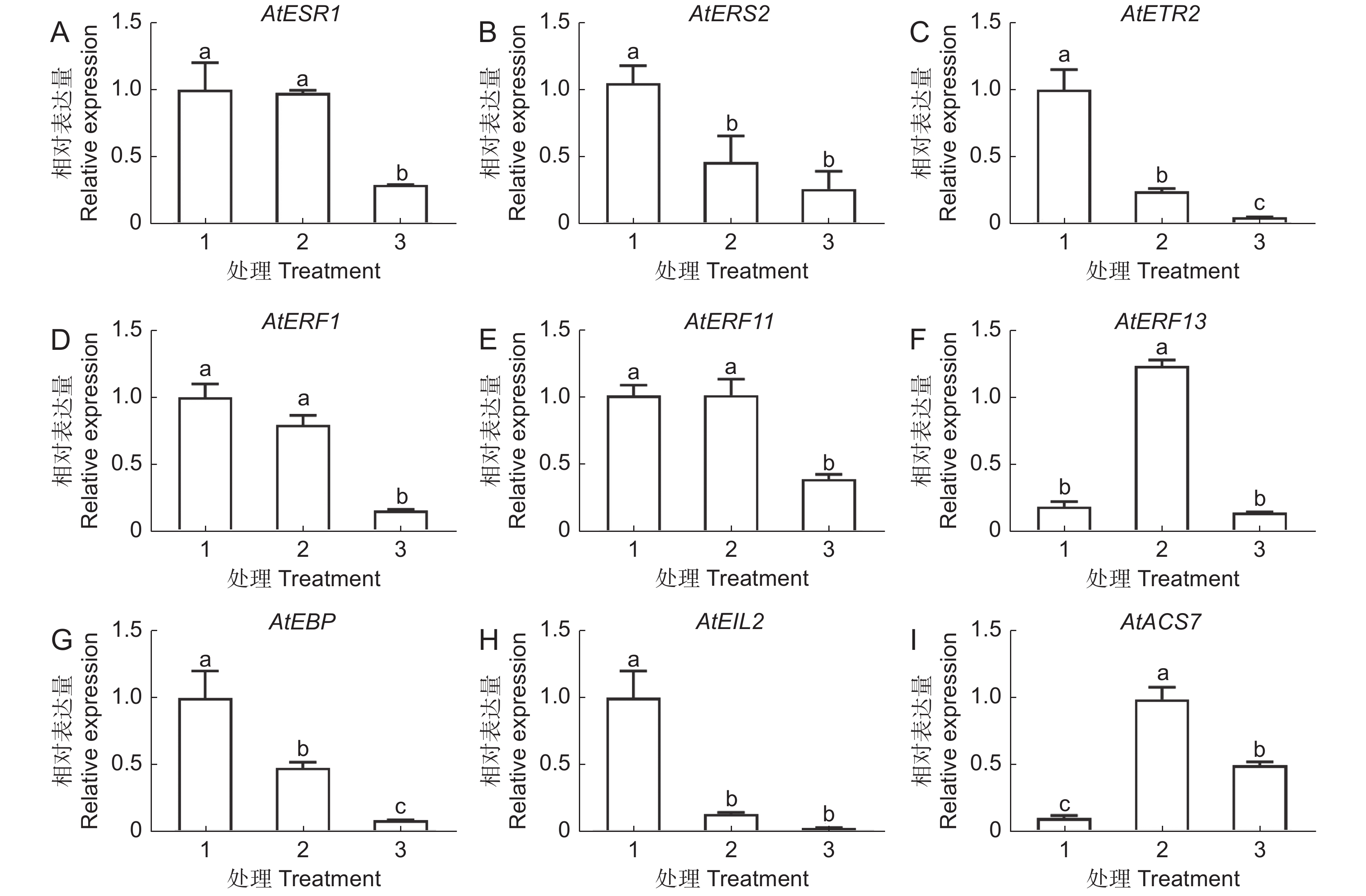
GO分析结果表明,30 mg/L AgNPs和30 mg/L Ag+均能显著影响根毛细胞分化和发育相关基因的表达。为了进一步验证转录组数据的准确性,选取了8个差异表达基因进行RT-qPCR分析(图6)。结果表明,两种处理均显著抑制根毛发育相关基因的表达,包括根毛特异表达基因(AtRHS12、AtRHS13、AtRHS14、AtRHS18)、根毛形态发生相关基因(AtMRH2、AtMRH6)、纤维素合成酶家族蛋白基因(AtCSLB05)和Sec14P样磷脂酰肌醇转移家族蛋白基因(AtCOW1)。此外,我们还发现,部分差异基因富集在乙烯调控反应中(附图2),因此选取9个与乙烯响应调控相关的基因进行RT-qPCR分析。结果表明,两种处理均显著下调4个乙烯调控相关基因的表达,包括AtEBP、AtERS2、AtETR2和AtEIL2。两种处理均可上调参与乙烯生物合成的1-氨基-环丙烷-1-羧酸合成酶7基因(AtACS7),但乙烯反应感受器1(AtERS1)、乙烯反应元件结合因子11(AtERF11)和乙烯反应元件结合因子1基因(AtERF1)仅在30 mg/L AgNPs处理后显著下调,而乙烯反应元件结合因子13(AtERF13)仅在30 mg/L Ag+处理时上调(图7)。
3. 讨论
3.1 AgNPs主要在拟南芥根中积累
根系是植物吸收水分和养分的重要器官,暴露在含有AgNPs的环境中后,环境中的AgNPs会吸附在根表皮表面,或通过根表皮进入并积累在柱细胞中[13, 18]。研究表明,AgNPs可以直接被植物根系吸收,然后通过维管组织向地上部转运[8, 35, 36]。本研究中,拟南芥根中积累的Ag含量显著高于叶片。前人研究表明,AgNPs处理后,拟南芥根中积累的Ag含量是叶片中的10倍[37];此外,与AgNO3相比,AgNPs处理后植物中积累的Ag含量较低[38]。但也有研究发现,无论是AgNPs还是AgNO3处理,烟草(Nicotiana tabacum L.)根部和叶片中Ag的积累量相同[39]。本研究发现,AgNPs处理的拟南芥根部积累的Ag比同等浓度的Ag+处理组更高。Qian等[40]也发现,与相同浓度的AgNO3相比,AgNPs处理后拟南芥叶片中的Ag含量更高。Nair和Chung[41]认为,暴露于AgNPs的植物中Ag积累较高的原因可能是植物可以直接吸收AgNPs,同时,AgNPs在根表面被氧化成Ag+后,也可直接进入根组织。
3.2 AgNPs破坏拟南芥根尖细胞活性,影响根系生长
前人研究表明,AgNPs对根系伸长的影响较小,而Ag+则明显抑制根系伸长[42, 43]。本研究也发现,30 mg/L AgNPs处理3 d和6 d后拟南芥主根长度与对照组没有显著差异,但30 mg/L Ag+则显著抑制主根伸长。AgNPs对植物根系生长的影响与AgNPs浓度、粒径和包被物等因素有关[44, 45]。AgNPs对拟南芥主根伸长的影响存在浓度依赖性,高浓度抑制伸长,而低浓度促进伸长[13]。也有研究发现,不同包被物的AgNPs对根系伸长的影响也不一样,柠檬酸盐包和PVP包被的AgNPs对洋葱根系伸长没有显著影响,但CTAB包被的AgNPs则显著抑制根系伸长[38]。因此,评估AgNPs对根系生长的影响需要考虑多种因素。根毛增加了根部吸收土壤中水分和营养物质的表面积,但其生长发育也容易受到环境胁迫的影响[27, 46]。研究表明,AgNPs和Ag+处理抑制拟南芥根毛的生长,破坏根尖分生组织的完整性[18]。本研究也发现,与对照组相比,30 mg/L的AgNPs和Ag+明显抑制拟南芥根毛的数量和长度,根尖表现出无根毛的表型,类似于一些“无毛”突变体[47]。此外,转录组测序和RT-qPCR结果也证明,根毛形成和分化中起关键作用的基因(AtRHS12、AtRHS13、AtRHS14、AtRHS18、AtMRH2、AtMRH6、AtCSLB05以及AtCOW1)均被AgNPs和Ag+显著抑制。García-Sánchez等[48]研究发现,碳纳米管、TiO2NPs和AgNPs可通过抑制拟南芥根毛特异基因的表达影响根毛发育,包括RHS12、RHS13、RHS15和RHS19等。因此,根毛特异基因的转录抑制可能是AgNPs和Ag+抑制拟南芥根毛生长发育,呈现“无根毛”表型的原因。
非生物胁迫可以诱导胼胝质沉积和根细胞损伤[49, 50]。胼胝质积累过多会扰乱或抑制韧皮部运输,从而阻碍根部生长所需光同化物质的供应,导致植物生长发育迟缓[34]。PI可以渗透死细胞的细胞膜,普遍用于检测根细胞活力[51]。本研究结果显示,30 mg/L 的AgNPs或Ag+处理后根系蓝色荧光和红色荧光强度均显著高于对照组,表明两种物质均明显诱导拟南芥根系胼胝质的沉积和细胞损伤。此外,GO富集分析也表明上述两种处理影响拟南芥根表皮细胞分化和细胞壁形成相关基因的表达。前人研究表明,在初生根尖生长过程中,细胞壁的完整性会受到快速扩张细胞的干扰,且该过程不可避免地会损失细胞壁的完整性。非生物胁迫与细胞壁完整性之间存在相互作用,从而影响植物初生根系的生长发育[50, 52, 53]。因此,AgNPs和Ag+引起的根部细胞损伤和胼胝质沉积可能导致根毛生长和发育受到抑制。此外,30 mg/L的 AgNPs或Ag+处理后,根系荧光强度显著高于0.12 mg/L Ag+处理组,表明AgNPs对根细胞损伤和胼胝质沉积的影响不仅仅是因为纳米颗粒的存在。
3.3 乙烯参与AgNPs对拟南芥根向重力性的影响
根向重力性是植物适应陆地环境最重要的过程之一[54],本研究中,AgNPs和Ag+处理均明显影响拟南芥的根负向重力性。植物通过激素可以改变根系形态,从而适应环境中的非生物胁迫[28, 29, 55]。Sun等[56]研究表明,AgNPs通过阻断生长素途径影响根向地性。根据Cholodny-Went理论,生长素虽然是控制根向重力性的主要因素,但仍需要考虑生长素与乙烯之间的相互作用[57]。此外,Nziengui等[58]发现叶酸前体对氨基苯甲酸(PABA)以乙烯依赖的方式促进拟南芥根系的向重力性。因此,乙烯可能也是影响植物根负向重力性的一种关键激素。本研究中,转录组测序和RT-qPCR均表明,AgNPs和Ag+明显影响乙烯生物合成和信号传导相关基因的表达,显著下调AtERS2、AtETR2、AtEBP和AtEIL2。此外,AgNPs和Ag+均显著上调AtACS7的表达。AtACS7是乙烯生物合成的限速酶基因,并以钙依赖的方式参与调控拟南芥根向重力性[27, 59]。Syu等[21]发现AgNPs可以抑制AtACS7的表达,表明AgNPs能够干扰乙烯的生物合成。以上结果表明,AgNPs和Ag+可能通过影响乙烯的生物合成或乙烯信号转导,从而调节拟南芥的根向重力性;但AgNPs和Ag+处理后主根的生长方式存在明显差异,前者处理后主根表现出明显的弯曲生长状态,而后者处理后主根明显左偏,表明AgNPs可能不是通过释放Ag+,而是通过纳米颗粒特有的效应调控根向重力性。但AgNPs和Ag+通过乙烯调控根向重力的机制仍需要进一步研究。
1 如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。2 如需查阅附图内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。3 1~3)如需查阅附件内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。 -
图 1 AgNPs或Ag + 处理6 d后叶片和根中银含量
1:CK;2:30 mg/L AgNPs;3:30 mg/L Ag+;4:0.12 mg/L Ag+。不同小写字母表示不同处理间差异显著(P<0.05),下同。
Figure 1. Silver content in Arabidopsis thaliana leaves and roots after six days of exposure to AgNPs or Ag+
1: CK; 2: 30 mg/L AgNPs; 3: 30 mg/L Ag+; 4: 0.12 mg/L Ag+. Different lowercase letters indicate significant differences at P<0.05 level between different treatments, same below.
-
[1] Samarajeewa AD,Velicogna JR,Princz JI,Subasinghe RM,Scroggins RP,Beaudette LA. Effect of silver nano-particles on soil microbial growth,activity and community diversity in a sandy loam soil[J]. Environ Pollut,2017,220:504−513. doi: 10.1016/j.envpol.2016.09.094
[2] Tortella GR,Rubilar O,Durán N,Diez MC,Martínez M,et al. Silver nanoparticles:toxicity in model organisms as an overview of its hazard for human health and the environment[J]. J Hazard Mater,2020,390:121974. doi: 10.1016/j.jhazmat.2019.121974
[3] Colman BP,Espinasse B,Richardson CJ,Matson CW,Lowry GV,et al. Emerging contaminant or an old toxin in disguise? Silver nanoparticle impacts on ecosystems[J]. Environ Sci Technol,2014,48(9):5229−5236. doi: 10.1021/es405454v
[4] Huang DY,Dang F,Huang YN,Chen N,Zhou DM. Uptake,translocation,and transformation of silver nanoparticles in plants[J]. Environ Sci:Nano,2022,9(1):12−39.
[5] Giese B,Klaessig F,Park B,Kaegi R,Steinfeldt M,et al. Risks,release and concentrations of engineered nanomaterial in the environment[J]. Sci Rep,2018,8(1):1565. doi: 10.1038/s41598-018-19275-4
[6] Kulikova NA. Silver nanoparticles in soil:input,transformation,and toxicity[J]. Eurasian Soil Sci,2021,54(3):352−365. doi: 10.1134/S1064229321030091
[7] Chen S,Yan X,Peralta-Videa JR,Su ZY,Hong J,Zhao LJ. Biological effects of AgNPs on crop plants:environmental implications and agricultural applications[J]. Environ Sci:Nano,2023,10(1):62−71.
[8] Wu J,Wang GY,Vijver MG,Bosker T,Peijnenburg WJGM. Foliar versus root exposure of AgNPs to lettuce:phytotoxicity,antioxidant responses and internal translocation[J]. Environ Pollut,2020,261:114117. doi: 10.1016/j.envpol.2020.114117
[9] Jiang HS,Qiu XN,Li GB,Li W,Yin LY. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza[J]. Environ Toxicol Chem,2014,33:1398−1405. doi: 10.1002/etc.2577
[10] Zhang CL,Jiang HS,Gu SP,Zhou XH,Lu ZW,et al. Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity[J]. Environ Pollut,2019,252:1539−1549. doi: 10.1016/j.envpol.2019.06.032
[11] Hu PG,An J,Faulkner MM,Wu HH,Li ZH,et al. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles[J]. ACS Nano,2020,14(7):7970−7986. doi: 10.1021/acsnano.9b09178
[12] Wang WW,Yuan LY,Zhou JZ,Zhu X,Liao ZY,et al. Inorganic carbon utilization:a target of silver nanoparticle toxicity on a submerged macrophyte[J]. Environ Pollut,2023,318:120906. doi: 10.1016/j.envpol.2022.120906
[13] Achari GA,Kowshik M. Recent developments on nanotechnology in agriculture:plant mineral nutrition,health,and interactions with soil microflora[J]. J Agric Food Chem,2018,66(33):8647−8661. doi: 10.1021/acs.jafc.8b00691
[14] Peng XH,Palma S,Fisher NS,Wong SS. Effect of morphology of ZnO nanostructures on their toxicity to marine algae[J]. Aquat Toxicol,2011,102(3-4):186−196. doi: 10.1016/j.aquatox.2011.01.014
[15] Ke MJ,Qu Q,Peijnenburg WJGM,Li XX,Zhang M,et al. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways[J]. Sci Total Environ,2018,644:1070−1079. doi: 10.1016/j.scitotenv.2018.07.061
[16] Babu K,Deepa MA,Shankar SG,Rai S. Effect of nano-silver on cell division and mitotic chromosomes:a prefatory siren[J]. Internet J Nanotechnol,2008,2(2).
[17] Kumari M,Mukherjee A,Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa[J]. Sci Total Environ,2009,407(19):5243−5246. doi: 10.1016/j.scitotenv.2009.06.024
[18] Geisler-Lee J,Wang Q,Yao Y,Zhang W,Geisler M,et al. Phytotoxicity,accumulation and transport of silver nanoparticles by Arabidopsis thaliana[J]. Nanotoxicology,2013,7(3):323−337. doi: 10.3109/17435390.2012.658094
[19] Cox A,Venkatachalam P,Sahi S,Sharma N. Reprint of:silver and titanium dioxide nanoparticle toxicity in plants:a review of current research[J]. Plant Physiol Biochem,2017,110:33−49. doi: 10.1016/j.plaphy.2016.08.007
[20] Wang J,Koo Y,Alexander A,Yang Y,Westerhof S,et al. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag + at sublethal concentrations[J]. Environ Sci Technol,2013,47(10):5442−5449. doi: 10.1021/es4004334
[21] Syu YY,Hung JH,Chen JC,Chuang HW. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression[J]. Plant Physiol Biochem,2014,83:57−64. doi: 10.1016/j.plaphy.2014.07.010
[22] Vinković T,Novák O,Strnad M,Goessler W,Jurašin DD,et al. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles[J]. Environ Res,2017,156:10−18. doi: 10.1016/j.envres.2017.03.015
[23] López-Herrera A,Avalos-Borja M,García-Nava JR,Trejo-Téllez LI,Alarcón A,et al. Interaction of silver nanoparticles with the aquatic fern Azolla filiculoides:root structure,particle distribution,and silver accumulation[J]. J Nanopart Res,2021,23(1):13. doi: 10.1007/s11051-020-05120-1
[24] Morita MT,Tasaka M. Gravity sensing and signaling[J]. Curr Opin Plant Biol,2004,7(6):712−718. doi: 10.1016/j.pbi.2004.09.001
[25] Moore I. Gravitropism:lateral thinking in auxin transport[J]. Curr Biol,2002,12(13):R452−R454. doi: 10.1016/S0960-9822(02)00943-0
[26] Heffron LM,Korban SS. Evaluation of ethylene mutant snapdragon lines for rooting,gravitropism,1-MCP,ethylene,and vase-life responses[J]. Sci Hortic,2022,304:111274. doi: 10.1016/j.scienta.2022.111274
[27] Luo XJ,Chen ZZ,Gao JP,Gong ZZ. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis[J]. Plant J,2014,79(1):44−55. doi: 10.1111/tpj.12534
[28] Rahman A,Takahashi M,Shibasaki K,Wu S,Inaba T,et al. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells[J]. Plant Cell,2010,22(6):1762−1776. doi: 10.1105/tpc.110.075317
[29] Pernisova M,Prat T,Grones P,Harustiakova D,Matonohova M,et al. Cytokinins influence root gravitropism via differential regulation of auxin transporter expression and localization in Arabidopsis[J]. New Phytol,2016,212(2):497−509. doi: 10.1111/nph.14049
[30] Dimkpa CO,McLean JE,Martineau N,Britt DW,Haverkamp R,Anderson AJ. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix[J]. Environ Sci Technol,2013,47(2):1082−1090. doi: 10.1021/es302973y
[31] Huang XT,Li Y,Chen K,Chen HY,Wang F,et al. NOM mitigates the phytotoxicity of AgNPs by regulating rice physiology,root cell wall components and root morphology[J]. Environ Pollut,2020,260:113942. doi: 10.1016/j.envpol.2020.113942
[32] Zhang LQ,Wang WX. Dominant role of silver ions in silver nanoparticle toxicity to a unicellular alga:evidence from luminogen imaging[J]. Environ Sci Technol,2019,53(1):494−502. doi: 10.1021/acs.est.8b04918
[33] Zhang N,Fan YX,Li C,Wang QM,Leksawasdi N,et al. Cell permeability and nuclear DNA staining by propidium iodide in basidiomycetous yeasts[J]. Appl Microbiol Biotechnol,2018,102(9):4183−4191. doi: 10.1007/s00253-018-8906-8
[34] Jones DL,Blancaflor EB,Kochian LV,Gilroy S. Spatial coordination of aluminium uptake,production of reactive oxygen species,callose production and wall rigidification in maize roots[J]. Plant Cell Environ,2006,29(7):1309−1318. doi: 10.1111/j.1365-3040.2006.01509.x
[35] Thuesombat P,Hannongbua S,Akasit S,Chadchawan S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth[J]. Ecotoxicol Environ Saf,2014,104:302−309. doi: 10.1016/j.ecoenv.2014.03.022
[36] Vannini C,Domingo G,Onelli E,De Mattia F,Bruni I,et al. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings[J]. J Plant Physiol,2014,171(13):1142−1148. doi: 10.1016/j.jplph.2014.05.002
[37] Geisler-Lee J,Brooks M,Gerfen JR,Wang Q,Fotis C,et al. Reproductive toxicity and life history study of silver nanoparticle effect,uptake and transport in Arabidopsis thaliana[J]. Nanomaterials,2014,4(2):301−318. doi: 10.3390/nano4020301
[38] Cvjetko P,Milošić A,Domijan AM,Vrček IV,Tolić S,et al. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots[J]. Ecotoxicol Environ Saf,2017,137:18−28. doi: 10.1016/j.ecoenv.2016.11.009
[39] Cvjetko P,Zovko M,Štefanić PP,Biba R,Tkalec M,et al. Phytotoxic effects of silver nanoparticles in tobacco plants[J]. Environ Sci Pollut Res,2018,25(6):5590−5602. doi: 10.1007/s11356-017-0928-8
[40] Qian HF,Peng XF,Han X,Ren J,Sun LW,Fu ZW. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana[J]. J Environ Sci,2013,25(9):1947−1956. doi: 10.1016/S1001-0742(12)60301-5
[41] Nair PMG,Chung IM. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings[J]. Chemosphere,2014,112:105−113. doi: 10.1016/j.chemosphere.2014.03.056
[42] Yasur J,Rani PU. Environmental effects of nanosilver:impact on castor seed germination,seedling growth,and plant physiology[J]. Environ Sci Pollut Res,2013,20(12):8636−8648. doi: 10.1007/s11356-013-1798-3
[43] Vannini C,Domingo G,Onelli E,Prinsi B,Marsoni M,et al. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate[J]. PLoS One,2013,8(7):e68752. doi: 10.1371/journal.pone.0068752
[44] Yin LY,Colman BP,McGill BM,Wright JP,Bernhardt ES. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants[J]. PLoS One,2012,7(10):e47674. doi: 10.1371/journal.pone.0047674
[45] Guo XQ,Li Y,Yan J,Ingle T,Jones MY,et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays[J]. Nanotoxicology,2016,10(9):1373−1384. doi: 10.1080/17435390.2016.1214764
[46] Ma Z,Bielenberg DG,Brown KM,Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana[J]. Plant Cell Environ,2001,24(4):459−467. doi: 10.1046/j.1365-3040.2001.00695.x
[47] Bruex A,Kainkaryam RM,Wieckowski Y,Kang YH,Bernhardt C,et al. A gene regulatory network for root epidermis cell differentiation in Arabidopsis[J]. PLoS Genet,2012,8(1):e1002446. doi: 10.1371/journal.pgen.1002446
[48] García-Sánchez S,Bernales I,Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling[J]. BMC Genomics,2015,16(1):341. doi: 10.1186/s12864-015-1530-4
[49] Liu J,Liu Y,Wang S,Cui YQ,Yan DW. Heat stress reduces root meristem size via induction of plasmodesmal callose accumulation inhibiting phloem unloading in Arabidopsis[J]. Int J Mol Sci,2022,23(4):2063. doi: 10.3390/ijms23042063
[50] Riaz M,Yan L,Wu XW,Hussain S,Aziz O,Jiang CC. Boron deprivation induced inhibition of root elongation is provoked by oxidative damage,root injuries and changes in cell wall structure[J]. Environ Exp Bot,2018,156:74−85. doi: 10.1016/j.envexpbot.2018.08.032
[51] Kwon YI,Abe K,Endo M,Osakabe K,Ohtsuki N,et al. DNA replication arrest leads to enhanced homologous recombination and cell death in meristems of rice OsRecQl4 mutants[J]. BMC Plant Biol,2013,13(1):62. doi: 10.1186/1471-2229-13-62
[52] Refrégier G,Pelletier S,Jaillard D,Höfte H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis[J]. Plant Physiol,2004,135(2):959−968. doi: 10.1104/pp.104.038711
[53] Tsang DL,Edmond C,Harrington JL,Nüshse TS. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent,ethylene-independent pathway[J]. Plant Physiol,2011,156(2):596−604. doi: 10.1104/pp.111.175372
[54] Nakamura M,Nishimura T,Morita MT. Gravity sensing and signal conversion in plant gravitropism[J]. J Exp Bot,2019,70(14):3495−3506. doi: 10.1093/jxb/erz158
[55] Li Y,Wang YP,Tan ST,Li Z,Yuan Z,et al. Root growth adaptation is mediated by PYLs ABA receptor-PP2A protein phosphatase complex[J]. Adv Sci,2020,7(3):1901455. doi: 10.1002/advs.201901455
[56] Sun JZ,Wang LK,Li S,Yin LY,Huang J,Chen CL. Toxicity of silver nanoparticles to Arabidopsis:inhibition of root gravitropism by interfering with auxin pathway[J]. Environ Toxicol Chem,2017,36(10):2773−2780. doi: 10.1002/etc.3833
[57] Santisree P,Nongmaithem S,Vasuki H,Sreelakshmi Y,Ivanchenko MG,Sharma R. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling[J]. Plant Physiol,2011,156(3):1424−1438. doi: 10.1104/pp.111.177014
[58] Nziengui H,Lasok H,Kochersperger P,Ruperti B,Rébeillé F,et al. Root gravitropism is regulated by a crosstalk between para-aminobenzoic acid,ethylene,and auxin[J]. Plant Physiol,2018,178(3):1370−1389. doi: 10.1104/pp.18.00126
[59] Huang SJ,Chang CL,Wang PH,Tsai MC,Hsu PH,Chang IF. A type Ⅲ ACC synthase,ACS7,is involved in root gravitropism in Arabidopsis thaliana[J]. J Exp Bot,2013,64(14):4343−4360. doi: 10.1093/jxb/ert241
-
其他相关附件
-
PDF格式
王颖 附件 点击下载(702KB)
-



 下载:
下载: