Mechanism underlying silver nanoparticle induction of root cell damage and negative gravitropism in main roots of Arabidopsis thaliana (L.) Heynh.
-
摘要:
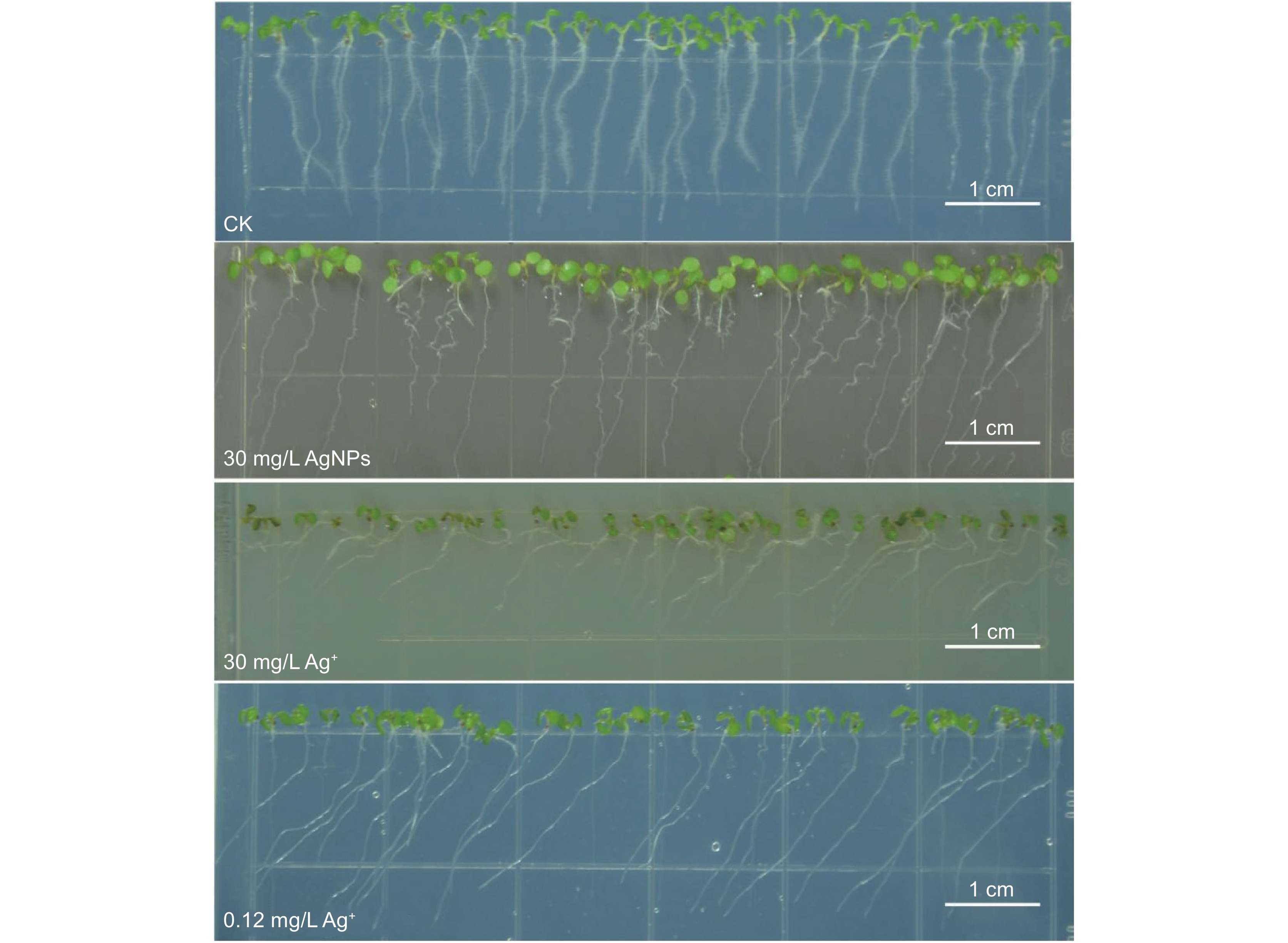
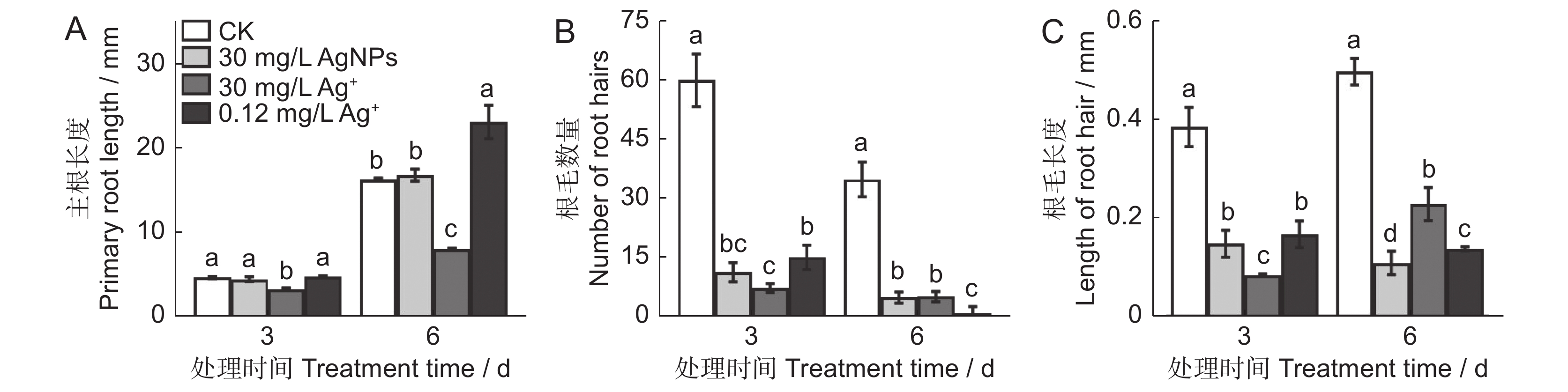
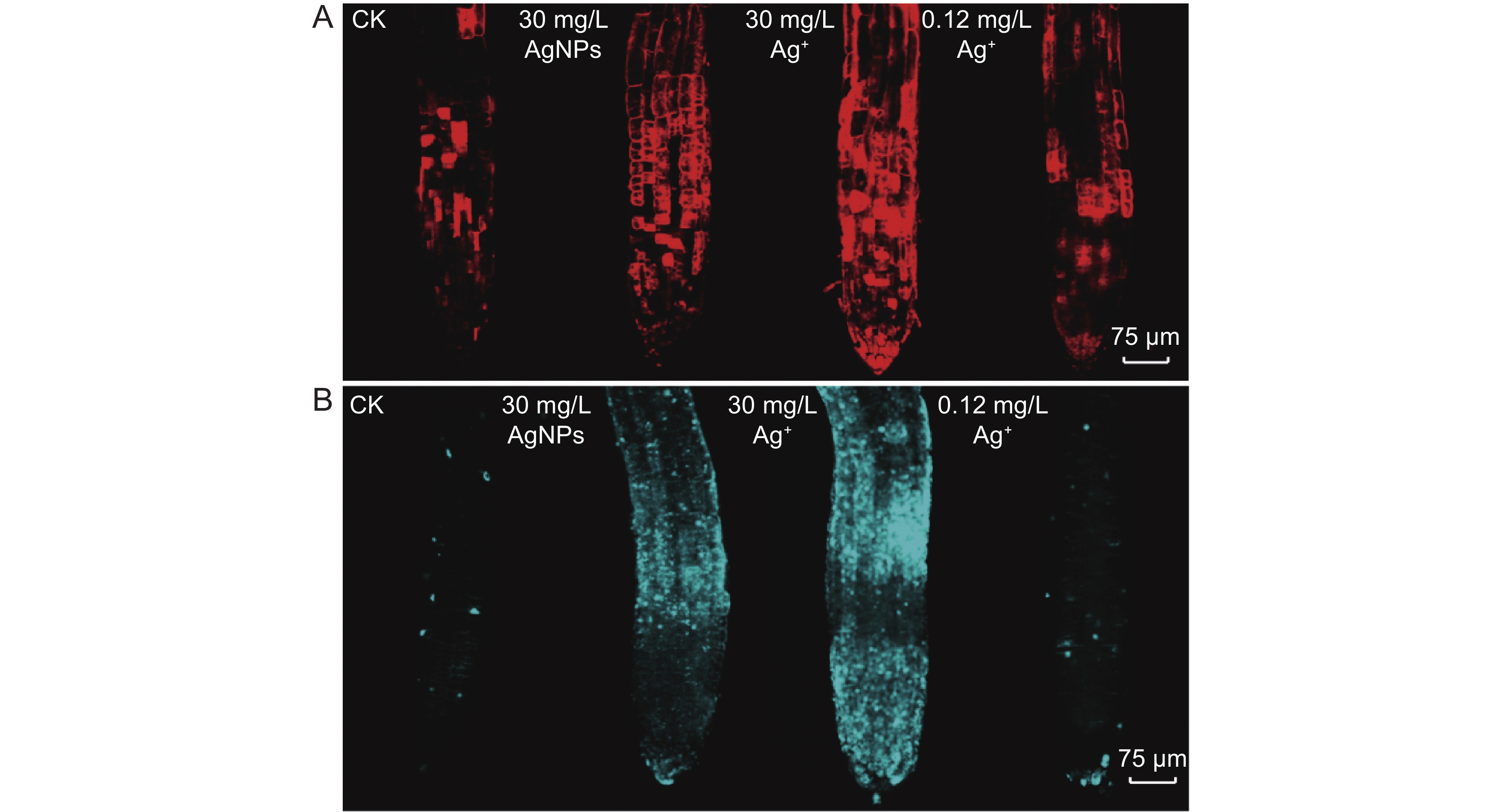
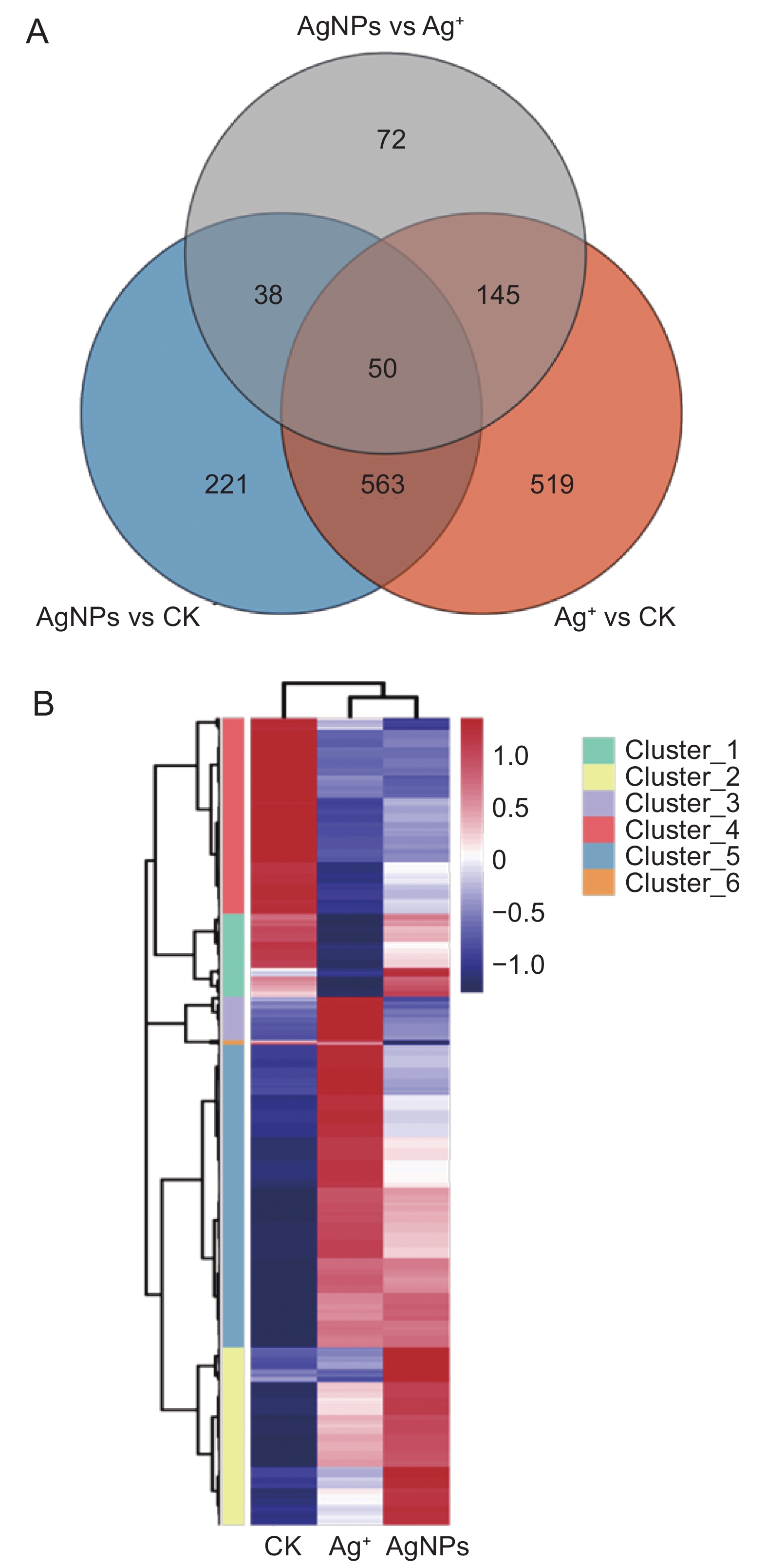
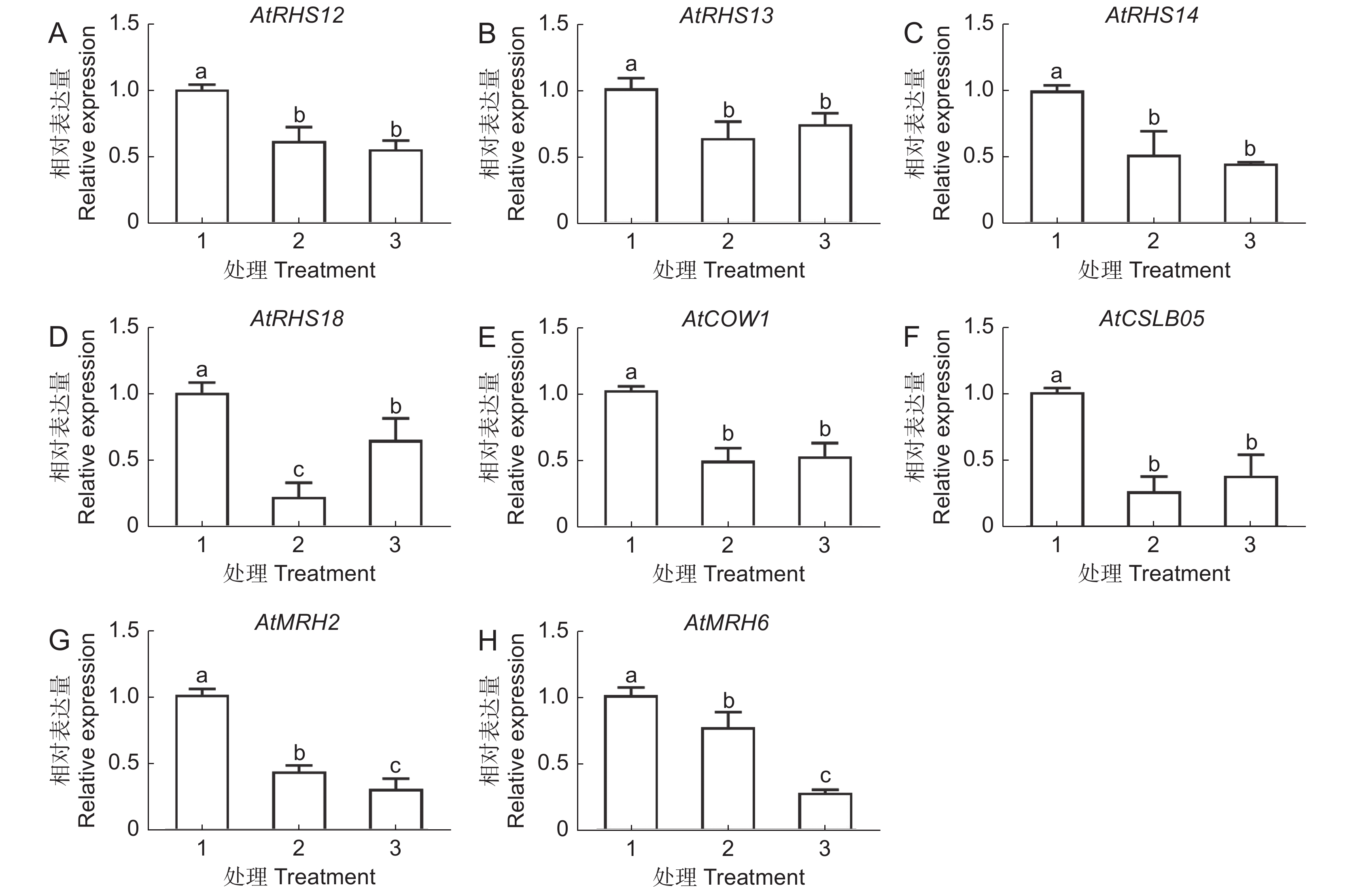
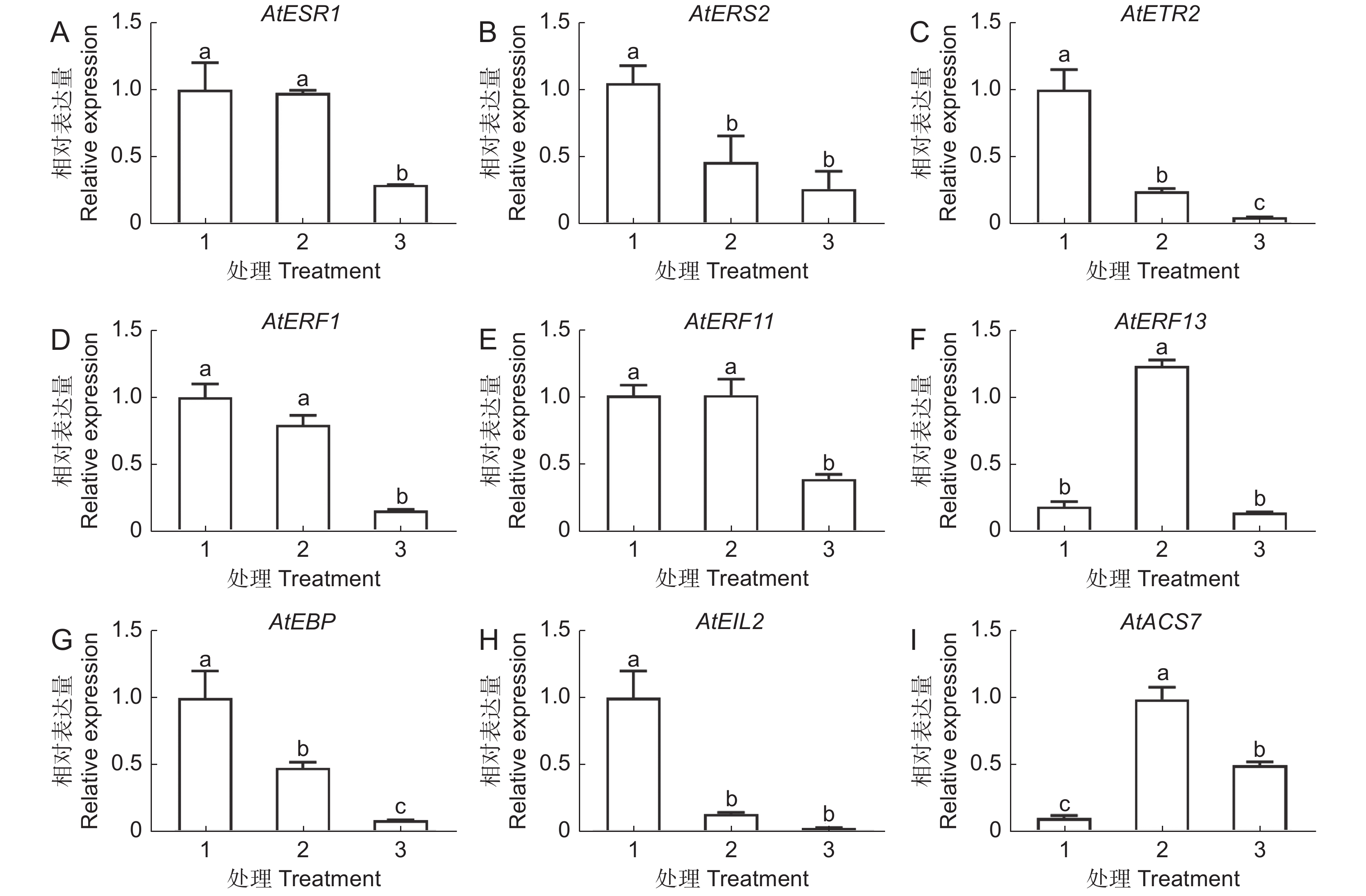
本研究以拟南芥(Arabidopsis thaliana (L.) Heynh.)为材料,分析了纳米银(AgNPs)对根系生长、根细胞损伤和主根负向重力性的影响。结果显示:(1)30 mg/L AgNPs处理后,拟南芥根系中积累了大量Ag元素,显著高于0.12 mg/L Ag+(30 mg/L AgNPs中释放的Ag+浓度)处理组。(2)30 mg/L AgNPs显著抑制根毛数量和根毛长度,并抑制根毛细胞发育相关基因AtRHS12、AtRHS14、AtCOW1和AtMRH12的表达。(3)30 mg/L的AgNPs或Ag+均能显著诱导根系胼胝质的形成,并损伤根细胞,而0.12 mg/L Ag+处理后根系损伤不明显。(4)30 mg/L AgNPs导致拟南芥主根呈螺旋状生长,而30 mg/L及0.12 mg/L的Ag+则引发主根向左偏移。转录组测序及RT-qPCR分析结果表明,30 mg/L的 AgNPs或Ag+均显著下调乙烯调控相关基因的表达,并上调乙烯合成关键酶基因AtACS7的表达。研究结果表明,30 mg/L AgNPs抑制拟南芥根毛生长,诱导根细胞损伤,并可能通过乙烯信号通路调控根的弯曲生长,其毒性效应可能来源于纳米银本身的颗粒效应。
Abstract:This study explored the effects of silver nanoparticles (AgNPs) on root growth, cellular integrity, and negative gravitropism in Arabidopsis thaliana (L.) Heynh.. Results revealed that: (1) Treatment with 30 mg/L AgNPs led to a significant accumulation of Ag in roots, surpassing the levels observed in roots treated with 0.12 mg/L Ag+ (equivalent to the Ag+ released from 30 mg/L AgNPs). (2) Exposure to 30 mg/L AgNPs markedly inhibited the number and length of root hairs. Transcriptome sequencing and RT-qPCR analyses indicated that 30 mg/L AgNPs suppressed the expression of key genes associated with root hair cell development, including AtRHS12, AtRHS14, AtCOW1, and AtMRH12. (3) Both 30 mg/L AgNPs and 30 mg/L Ag+ induced root callus formation and caused significant root cell damage in A. thaliana. However, no significant root damage was observed in plants treated with 0.12 mg/L Ag+, suggesting that the Ag+ released from AgNPs was insufficient to cause cellular damage. (4) Exposure to 30 mg/L AgNPs induced a spiral growth pattern in the main root, contrasting with the leftward growth induced by 30 mg/L and 0.12 mg/L Ag+. Transcriptome sequencing and RT-qPCR analyses revealed that both 30 mg/L AgNPs and 30 mg/L Ag+ significantly down-regulated ethylene-regulated genes such as AtERS1, AtETR2, AtERF1, AtERF11, and AtEBP, while up-regulating the key ethylene synthesis gene AtACS7. These findings suggest that AgNPs and Ag+ may influence negative gravitropism in A. thaliana roots by modulating ethylene signaling pathways and ethylene biosynthesis. In conclusion, 30 mg/L AgNPs inhibit root hair growth, induce root cell damage, and influence gravitropic bending in A. thaliana roots through the ethylene signaling pathway. The observed toxic effects are likely attributable to the intrinsic properties of the nanoparticles.
-
Keywords:
- Silver nanoparticles /
- Root negative gravitropism /
- Ethylene /
- Arabidopsis thaliana
-
1 如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。2 如需查阅附图内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。3 1~3)如需查阅附件内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。 -
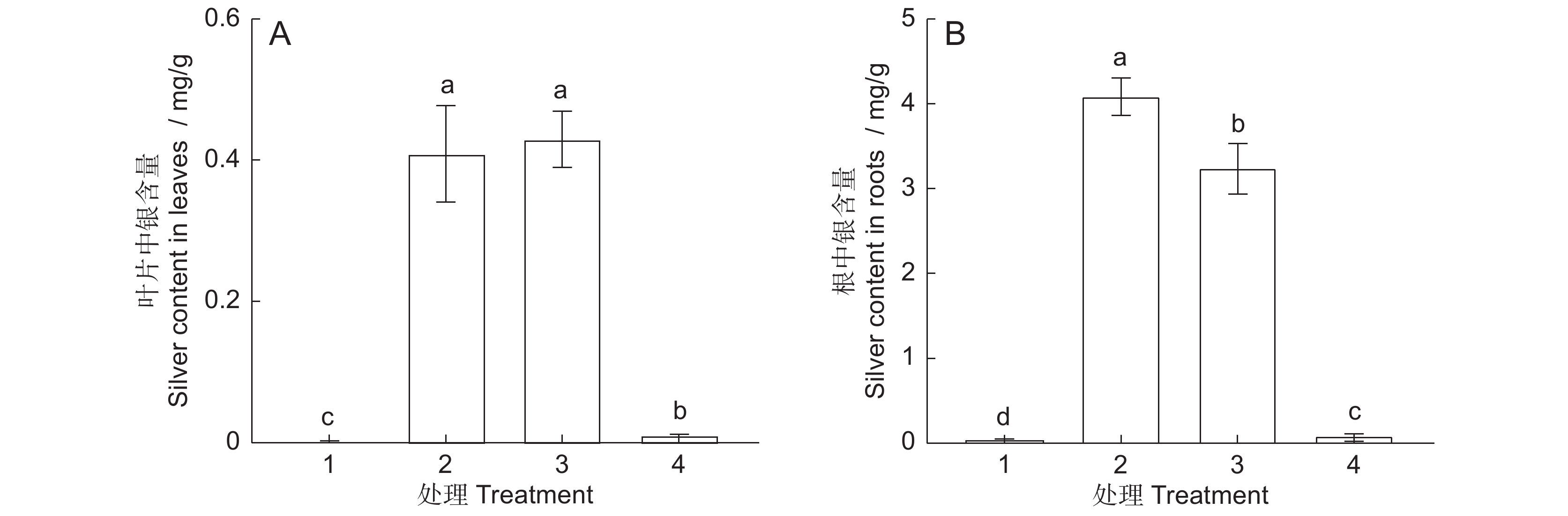
图 1 AgNPs或Ag + 处理6 d后叶片和根中银含量
1:CK;2:30 mg/L AgNPs;3:30 mg/L Ag+;4:0.12 mg/L Ag+。不同小写字母表示不同处理间差异显著(P<0.05),下同。
Figure 1. Silver content in Arabidopsis thaliana leaves and roots after six days of exposure to AgNPs or Ag+
1: CK; 2: 30 mg/L AgNPs; 3: 30 mg/L Ag+; 4: 0.12 mg/L Ag+. Different lowercase letters indicate significant differences at P<0.05 level between different treatments, same below.
-
[1] Samarajeewa AD,Velicogna JR,Princz JI,Subasinghe RM,Scroggins RP,Beaudette LA. Effect of silver nano-particles on soil microbial growth,activity and community diversity in a sandy loam soil[J]. Environ Pollut,2017,220:504−513. doi: 10.1016/j.envpol.2016.09.094
[2] Tortella GR,Rubilar O,Durán N,Diez MC,Martínez M,et al. Silver nanoparticles:toxicity in model organisms as an overview of its hazard for human health and the environment[J]. J Hazard Mater,2020,390:121974. doi: 10.1016/j.jhazmat.2019.121974
[3] Colman BP,Espinasse B,Richardson CJ,Matson CW,Lowry GV,et al. Emerging contaminant or an old toxin in disguise? Silver nanoparticle impacts on ecosystems[J]. Environ Sci Technol,2014,48(9):5229−5236. doi: 10.1021/es405454v
[4] Huang DY,Dang F,Huang YN,Chen N,Zhou DM. Uptake,translocation,and transformation of silver nanoparticles in plants[J]. Environ Sci:Nano,2022,9(1):12−39.
[5] Giese B,Klaessig F,Park B,Kaegi R,Steinfeldt M,et al. Risks,release and concentrations of engineered nanomaterial in the environment[J]. Sci Rep,2018,8(1):1565. doi: 10.1038/s41598-018-19275-4
[6] Kulikova NA. Silver nanoparticles in soil:input,transformation,and toxicity[J]. Eurasian Soil Sci,2021,54(3):352−365. doi: 10.1134/S1064229321030091
[7] Chen S,Yan X,Peralta-Videa JR,Su ZY,Hong J,Zhao LJ. Biological effects of AgNPs on crop plants:environmental implications and agricultural applications[J]. Environ Sci:Nano,2023,10(1):62−71.
[8] Wu J,Wang GY,Vijver MG,Bosker T,Peijnenburg WJGM. Foliar versus root exposure of AgNPs to lettuce:phytotoxicity,antioxidant responses and internal translocation[J]. Environ Pollut,2020,261:114117. doi: 10.1016/j.envpol.2020.114117
[9] Jiang HS,Qiu XN,Li GB,Li W,Yin LY. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza[J]. Environ Toxicol Chem,2014,33:1398−1405. doi: 10.1002/etc.2577
[10] Zhang CL,Jiang HS,Gu SP,Zhou XH,Lu ZW,et al. Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity[J]. Environ Pollut,2019,252:1539−1549. doi: 10.1016/j.envpol.2019.06.032
[11] Hu PG,An J,Faulkner MM,Wu HH,Li ZH,et al. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles[J]. ACS Nano,2020,14(7):7970−7986. doi: 10.1021/acsnano.9b09178
[12] Wang WW,Yuan LY,Zhou JZ,Zhu X,Liao ZY,et al. Inorganic carbon utilization:a target of silver nanoparticle toxicity on a submerged macrophyte[J]. Environ Pollut,2023,318:120906. doi: 10.1016/j.envpol.2022.120906
[13] Achari GA,Kowshik M. Recent developments on nanotechnology in agriculture:plant mineral nutrition,health,and interactions with soil microflora[J]. J Agric Food Chem,2018,66(33):8647−8661. doi: 10.1021/acs.jafc.8b00691
[14] Peng XH,Palma S,Fisher NS,Wong SS. Effect of morphology of ZnO nanostructures on their toxicity to marine algae[J]. Aquat Toxicol,2011,102(3-4):186−196. doi: 10.1016/j.aquatox.2011.01.014
[15] Ke MJ,Qu Q,Peijnenburg WJGM,Li XX,Zhang M,et al. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways[J]. Sci Total Environ,2018,644:1070−1079. doi: 10.1016/j.scitotenv.2018.07.061
[16] Babu K,Deepa MA,Shankar SG,Rai S. Effect of nano-silver on cell division and mitotic chromosomes:a prefatory siren[J]. Internet J Nanotechnol,2008,2(2).
[17] Kumari M,Mukherjee A,Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa[J]. Sci Total Environ,2009,407(19):5243−5246. doi: 10.1016/j.scitotenv.2009.06.024
[18] Geisler-Lee J,Wang Q,Yao Y,Zhang W,Geisler M,et al. Phytotoxicity,accumulation and transport of silver nanoparticles by Arabidopsis thaliana[J]. Nanotoxicology,2013,7(3):323−337. doi: 10.3109/17435390.2012.658094
[19] Cox A,Venkatachalam P,Sahi S,Sharma N. Reprint of:silver and titanium dioxide nanoparticle toxicity in plants:a review of current research[J]. Plant Physiol Biochem,2017,110:33−49. doi: 10.1016/j.plaphy.2016.08.007
[20] Wang J,Koo Y,Alexander A,Yang Y,Westerhof S,et al. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag + at sublethal concentrations[J]. Environ Sci Technol,2013,47(10):5442−5449. doi: 10.1021/es4004334
[21] Syu YY,Hung JH,Chen JC,Chuang HW. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression[J]. Plant Physiol Biochem,2014,83:57−64. doi: 10.1016/j.plaphy.2014.07.010
[22] Vinković T,Novák O,Strnad M,Goessler W,Jurašin DD,et al. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles[J]. Environ Res,2017,156:10−18. doi: 10.1016/j.envres.2017.03.015
[23] López-Herrera A,Avalos-Borja M,García-Nava JR,Trejo-Téllez LI,Alarcón A,et al. Interaction of silver nanoparticles with the aquatic fern Azolla filiculoides:root structure,particle distribution,and silver accumulation[J]. J Nanopart Res,2021,23(1):13. doi: 10.1007/s11051-020-05120-1
[24] Morita MT,Tasaka M. Gravity sensing and signaling[J]. Curr Opin Plant Biol,2004,7(6):712−718. doi: 10.1016/j.pbi.2004.09.001
[25] Moore I. Gravitropism:lateral thinking in auxin transport[J]. Curr Biol,2002,12(13):R452−R454. doi: 10.1016/S0960-9822(02)00943-0
[26] Heffron LM,Korban SS. Evaluation of ethylene mutant snapdragon lines for rooting,gravitropism,1-MCP,ethylene,and vase-life responses[J]. Sci Hortic,2022,304:111274. doi: 10.1016/j.scienta.2022.111274
[27] Luo XJ,Chen ZZ,Gao JP,Gong ZZ. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis[J]. Plant J,2014,79(1):44−55. doi: 10.1111/tpj.12534
[28] Rahman A,Takahashi M,Shibasaki K,Wu S,Inaba T,et al. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells[J]. Plant Cell,2010,22(6):1762−1776. doi: 10.1105/tpc.110.075317
[29] Pernisova M,Prat T,Grones P,Harustiakova D,Matonohova M,et al. Cytokinins influence root gravitropism via differential regulation of auxin transporter expression and localization in Arabidopsis[J]. New Phytol,2016,212(2):497−509. doi: 10.1111/nph.14049
[30] Dimkpa CO,McLean JE,Martineau N,Britt DW,Haverkamp R,Anderson AJ. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix[J]. Environ Sci Technol,2013,47(2):1082−1090. doi: 10.1021/es302973y
[31] Huang XT,Li Y,Chen K,Chen HY,Wang F,et al. NOM mitigates the phytotoxicity of AgNPs by regulating rice physiology,root cell wall components and root morphology[J]. Environ Pollut,2020,260:113942. doi: 10.1016/j.envpol.2020.113942
[32] Zhang LQ,Wang WX. Dominant role of silver ions in silver nanoparticle toxicity to a unicellular alga:evidence from luminogen imaging[J]. Environ Sci Technol,2019,53(1):494−502. doi: 10.1021/acs.est.8b04918
[33] Zhang N,Fan YX,Li C,Wang QM,Leksawasdi N,et al. Cell permeability and nuclear DNA staining by propidium iodide in basidiomycetous yeasts[J]. Appl Microbiol Biotechnol,2018,102(9):4183−4191. doi: 10.1007/s00253-018-8906-8
[34] Jones DL,Blancaflor EB,Kochian LV,Gilroy S. Spatial coordination of aluminium uptake,production of reactive oxygen species,callose production and wall rigidification in maize roots[J]. Plant Cell Environ,2006,29(7):1309−1318. doi: 10.1111/j.1365-3040.2006.01509.x
[35] Thuesombat P,Hannongbua S,Akasit S,Chadchawan S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth[J]. Ecotoxicol Environ Saf,2014,104:302−309. doi: 10.1016/j.ecoenv.2014.03.022
[36] Vannini C,Domingo G,Onelli E,De Mattia F,Bruni I,et al. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings[J]. J Plant Physiol,2014,171(13):1142−1148. doi: 10.1016/j.jplph.2014.05.002
[37] Geisler-Lee J,Brooks M,Gerfen JR,Wang Q,Fotis C,et al. Reproductive toxicity and life history study of silver nanoparticle effect,uptake and transport in Arabidopsis thaliana[J]. Nanomaterials,2014,4(2):301−318. doi: 10.3390/nano4020301
[38] Cvjetko P,Milošić A,Domijan AM,Vrček IV,Tolić S,et al. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots[J]. Ecotoxicol Environ Saf,2017,137:18−28. doi: 10.1016/j.ecoenv.2016.11.009
[39] Cvjetko P,Zovko M,Štefanić PP,Biba R,Tkalec M,et al. Phytotoxic effects of silver nanoparticles in tobacco plants[J]. Environ Sci Pollut Res,2018,25(6):5590−5602. doi: 10.1007/s11356-017-0928-8
[40] Qian HF,Peng XF,Han X,Ren J,Sun LW,Fu ZW. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana[J]. J Environ Sci,2013,25(9):1947−1956. doi: 10.1016/S1001-0742(12)60301-5
[41] Nair PMG,Chung IM. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings[J]. Chemosphere,2014,112:105−113. doi: 10.1016/j.chemosphere.2014.03.056
[42] Yasur J,Rani PU. Environmental effects of nanosilver:impact on castor seed germination,seedling growth,and plant physiology[J]. Environ Sci Pollut Res,2013,20(12):8636−8648. doi: 10.1007/s11356-013-1798-3
[43] Vannini C,Domingo G,Onelli E,Prinsi B,Marsoni M,et al. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate[J]. PLoS One,2013,8(7):e68752. doi: 10.1371/journal.pone.0068752
[44] Yin LY,Colman BP,McGill BM,Wright JP,Bernhardt ES. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants[J]. PLoS One,2012,7(10):e47674. doi: 10.1371/journal.pone.0047674
[45] Guo XQ,Li Y,Yan J,Ingle T,Jones MY,et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays[J]. Nanotoxicology,2016,10(9):1373−1384. doi: 10.1080/17435390.2016.1214764
[46] Ma Z,Bielenberg DG,Brown KM,Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana[J]. Plant Cell Environ,2001,24(4):459−467. doi: 10.1046/j.1365-3040.2001.00695.x
[47] Bruex A,Kainkaryam RM,Wieckowski Y,Kang YH,Bernhardt C,et al. A gene regulatory network for root epidermis cell differentiation in Arabidopsis[J]. PLoS Genet,2012,8(1):e1002446. doi: 10.1371/journal.pgen.1002446
[48] García-Sánchez S,Bernales I,Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling[J]. BMC Genomics,2015,16(1):341. doi: 10.1186/s12864-015-1530-4
[49] Liu J,Liu Y,Wang S,Cui YQ,Yan DW. Heat stress reduces root meristem size via induction of plasmodesmal callose accumulation inhibiting phloem unloading in Arabidopsis[J]. Int J Mol Sci,2022,23(4):2063. doi: 10.3390/ijms23042063
[50] Riaz M,Yan L,Wu XW,Hussain S,Aziz O,Jiang CC. Boron deprivation induced inhibition of root elongation is provoked by oxidative damage,root injuries and changes in cell wall structure[J]. Environ Exp Bot,2018,156:74−85. doi: 10.1016/j.envexpbot.2018.08.032
[51] Kwon YI,Abe K,Endo M,Osakabe K,Ohtsuki N,et al. DNA replication arrest leads to enhanced homologous recombination and cell death in meristems of rice OsRecQl4 mutants[J]. BMC Plant Biol,2013,13(1):62. doi: 10.1186/1471-2229-13-62
[52] Refrégier G,Pelletier S,Jaillard D,Höfte H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis[J]. Plant Physiol,2004,135(2):959−968. doi: 10.1104/pp.104.038711
[53] Tsang DL,Edmond C,Harrington JL,Nüshse TS. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent,ethylene-independent pathway[J]. Plant Physiol,2011,156(2):596−604. doi: 10.1104/pp.111.175372
[54] Nakamura M,Nishimura T,Morita MT. Gravity sensing and signal conversion in plant gravitropism[J]. J Exp Bot,2019,70(14):3495−3506. doi: 10.1093/jxb/erz158
[55] Li Y,Wang YP,Tan ST,Li Z,Yuan Z,et al. Root growth adaptation is mediated by PYLs ABA receptor-PP2A protein phosphatase complex[J]. Adv Sci,2020,7(3):1901455. doi: 10.1002/advs.201901455
[56] Sun JZ,Wang LK,Li S,Yin LY,Huang J,Chen CL. Toxicity of silver nanoparticles to Arabidopsis:inhibition of root gravitropism by interfering with auxin pathway[J]. Environ Toxicol Chem,2017,36(10):2773−2780. doi: 10.1002/etc.3833
[57] Santisree P,Nongmaithem S,Vasuki H,Sreelakshmi Y,Ivanchenko MG,Sharma R. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling[J]. Plant Physiol,2011,156(3):1424−1438. doi: 10.1104/pp.111.177014
[58] Nziengui H,Lasok H,Kochersperger P,Ruperti B,Rébeillé F,et al. Root gravitropism is regulated by a crosstalk between para-aminobenzoic acid,ethylene,and auxin[J]. Plant Physiol,2018,178(3):1370−1389. doi: 10.1104/pp.18.00126
[59] Huang SJ,Chang CL,Wang PH,Tsai MC,Hsu PH,Chang IF. A type Ⅲ ACC synthase,ACS7,is involved in root gravitropism in Arabidopsis thaliana[J]. J Exp Bot,2013,64(14):4343−4360. doi: 10.1093/jxb/ert241
-
其他相关附件
-
PDF格式
王颖 附件 点击下载(702KB)
-



 下载:
下载: