Metabolomic analysis of the effects of MeJA on antioxidant compound synthesis in Lavandula angustifolia Mill. cells
-
摘要:
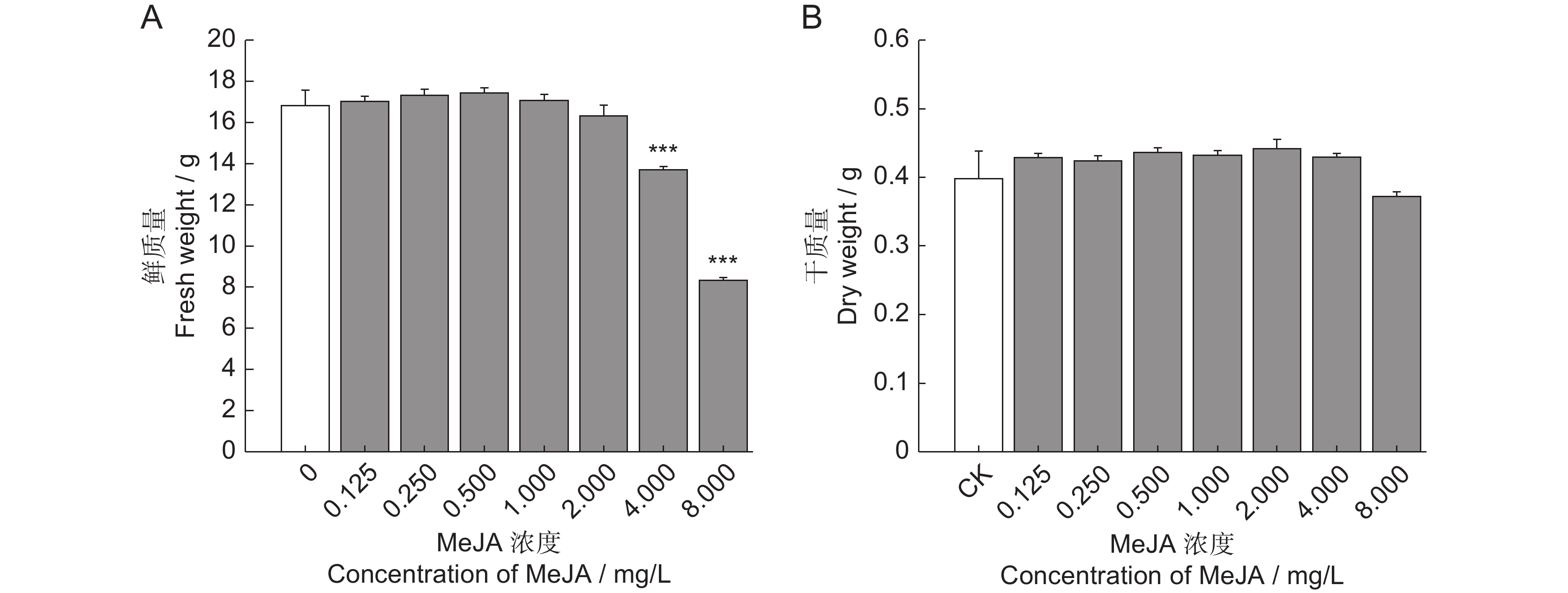
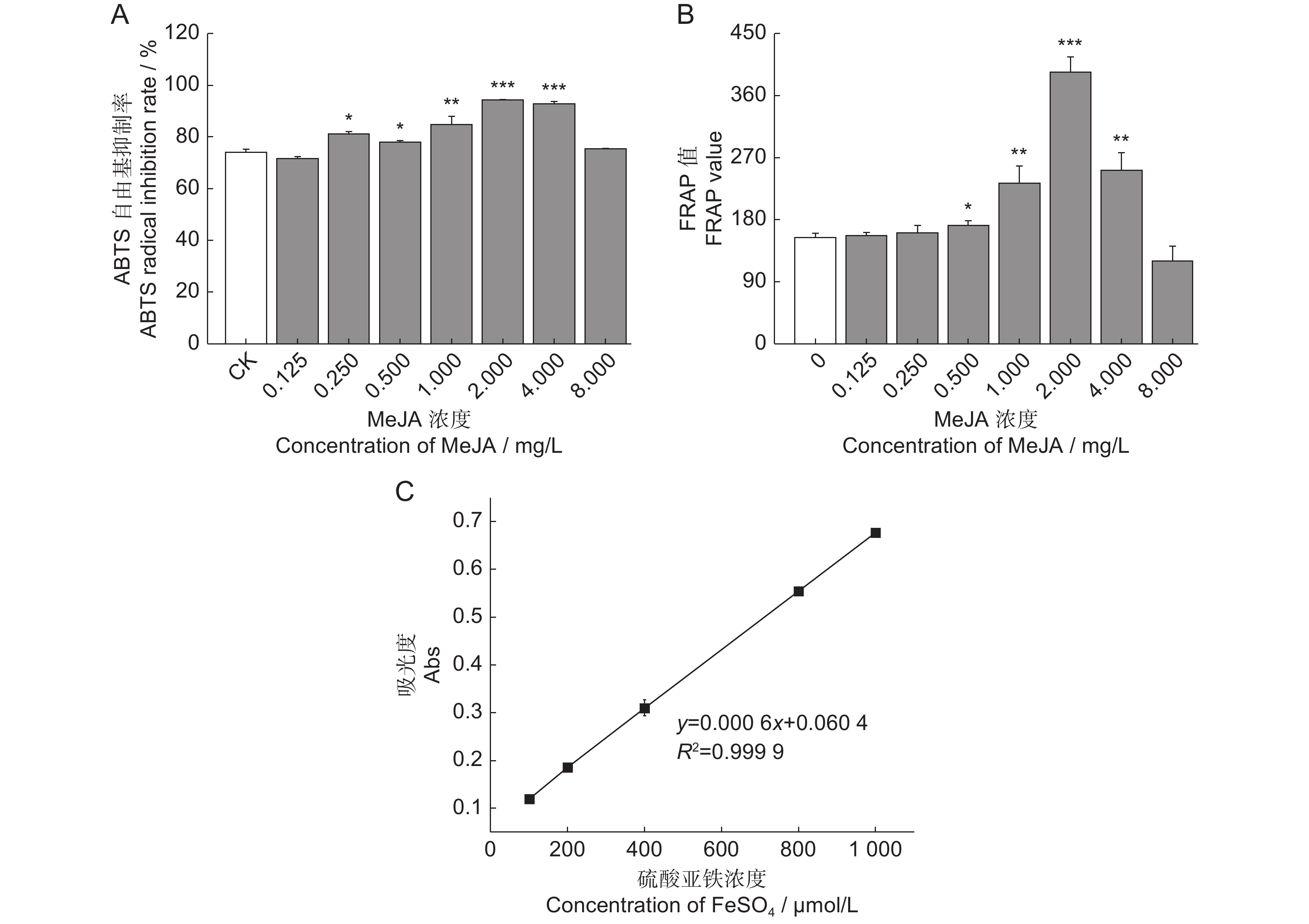
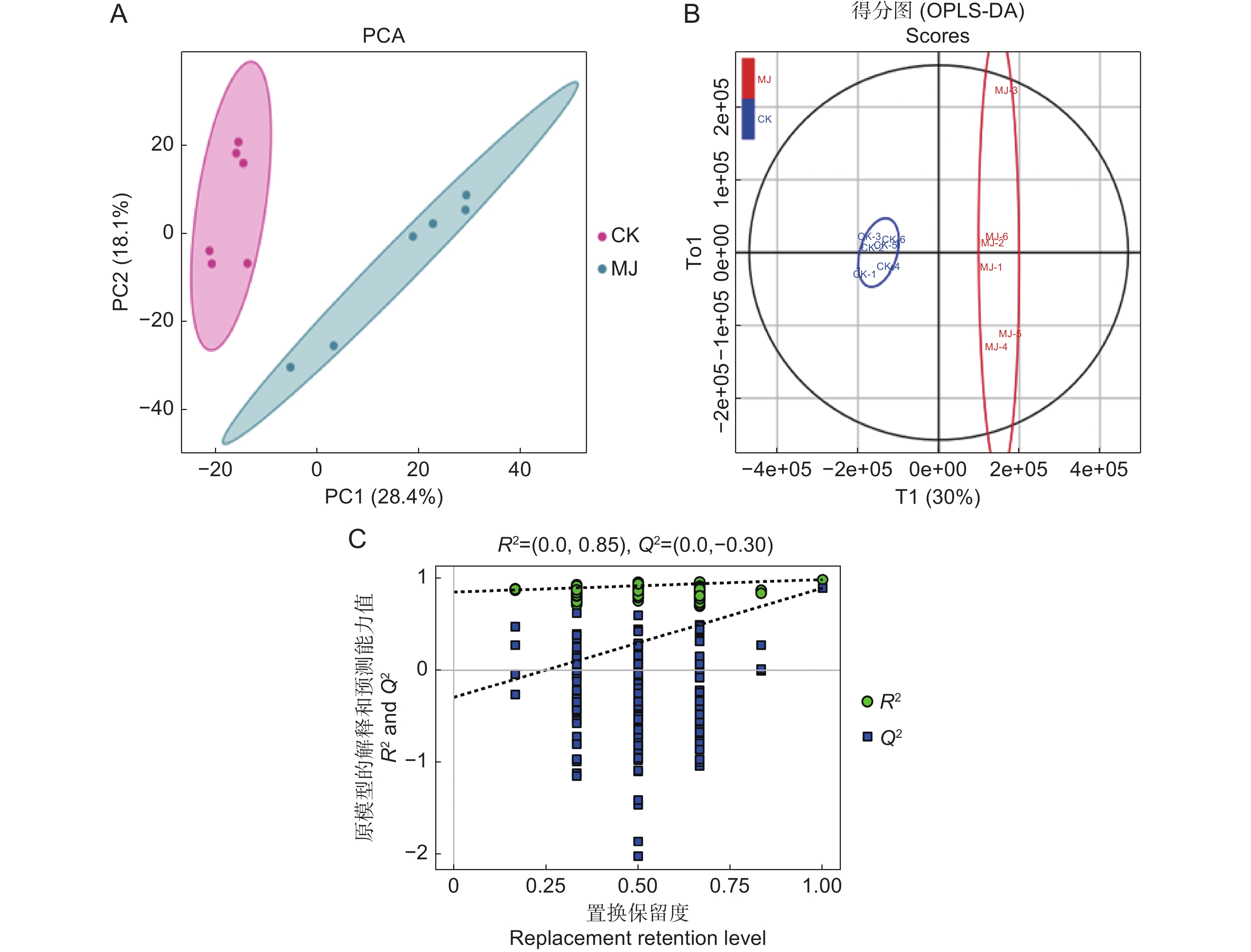
为了探究茉莉酸甲酯(MeJA)对薰衣草(Lavandula angustifolia Mill.)叶片悬浮细胞中抗氧化活性物质合成的影响,采用不同浓度的MeJA处理悬浮细胞,筛选出能够促进抗氧化物质合成的最佳条件,并通过非靶向代谢组学技术分析了MeJA对细胞代谢物合成的作用。结果显示:8 mg/L的MeJA显著抑制了薰衣草叶片悬浮细胞的生长和抗氧化活性;而2 mg/L的MeJA显著促进了抗氧化活性物的合成;与对照组相比,ABTS自由基清除率和FRAP值分别提高了1.30和2.56倍,总酚、总黄酮和总三萜含量分别增加了1.45、1.59和1.24倍。利用超高效液相色谱-四极杆飞行时间质谱(UHPLC-Q-TOF-MS)技术,对未处理组(CK)和2 mg/L MeJA处理组(MJ)进行代谢组学分析,共鉴定出1 403种代谢物,其中151种在两组间有显著差异。差异代谢物涉及177条代谢通路,主要富集于代谢信号转导通路和ABC转运蛋白通路。因此,MeJA可能主要通过影响代谢信号转导和调节ABC转运蛋白的功能影响薰衣草悬浮细胞中抗氧化活性物质的合成。
Abstract:The effects of methyl jasmonate (MeJA) on the synthesis of antioxidant compounds in Lavandula angustifolia Mill. leaf suspension cells were investigated by introducing varying concentrations of MeJA into the culture medium. The optimal conditions for enhancing antioxidant synthesis were determined, and non-targeted metabolomics was employed to analyze the impact of MeJA on metabolite production. Results demonstrated that 8 mg/L MeJA inhibited both cell growth and antioxidant activity, whereas 2 mg/L MeJA significantly promoted the biosynthesis of antioxidant compounds. Compared with the untreated group, cells treated with 2 mg/L MeJA exhibited a 1.30-fold increase in ABTS radical scavenging activity and a 2.56-fold increase in ferric reducing antioxidant power (FRAP). Additionally, total phenol, flavonoid, and triterpene contents were elevated by 1.45-fold, 1.59-fold, and 1.24-fold, respectively. Metabolomic profiling using UHPLC-Q-TOF-MS identified 1 403 metabolites, with 151 showing significant differences between untreated and MeJA-treated cells. These differential metabolites were associated with 177 metabolic pathways, predominantly enriched in metabolic pathways and ABC transporter pathways. These findings suggest that MeJA modulates antioxidant biosynthesis in L. angustifolia suspension cells primarily by influencing metabolic signal transduction and regulating ABC transporter activity.
-
Keywords:
- Lavandula angustifolia /
- Suspension culture /
- MeJA /
- Antioxidant /
- Metabolomics
-
-
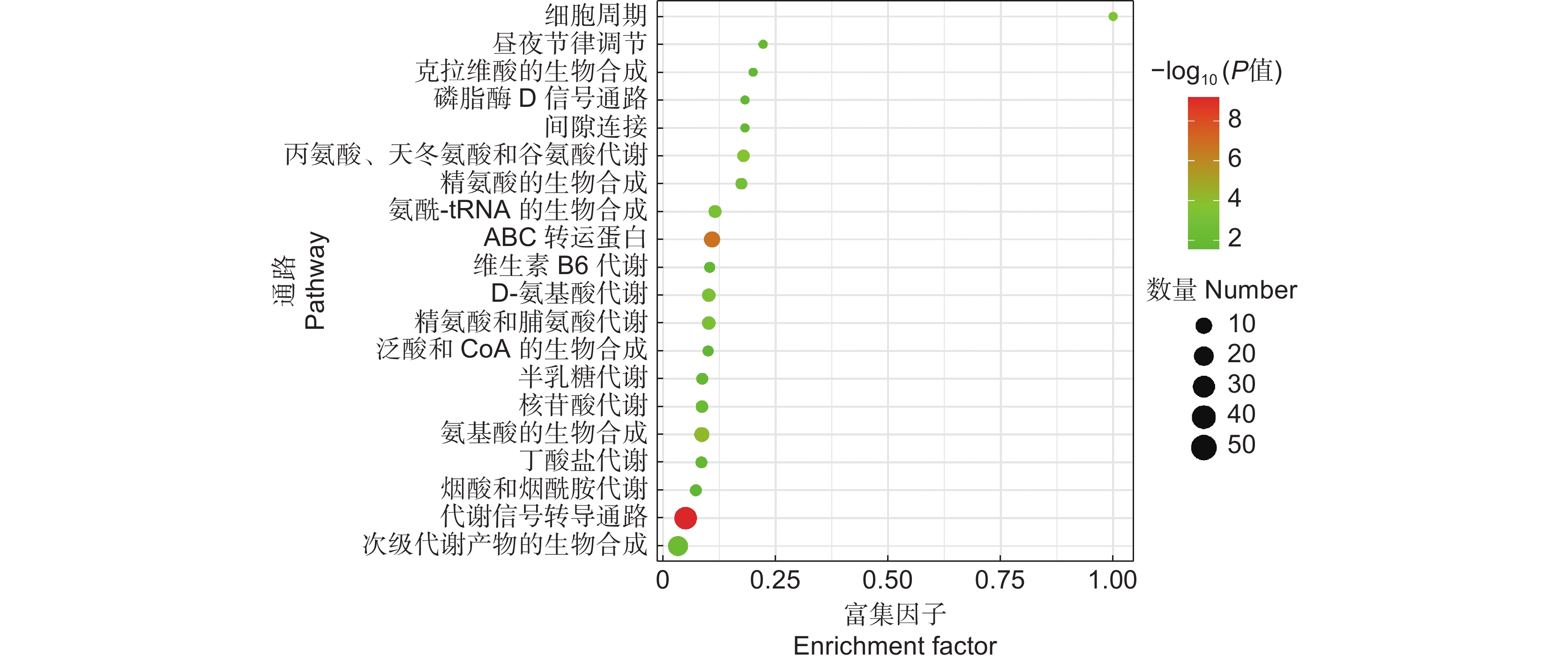
图 7 CK和MJ的差异代谢物KEGG通路富集
气泡越大,表示该通路中富集到的差异代谢物数量越多;颜色越红,表示差异代谢物在该通路上的富集越显著。
Figure 7. KEGG pathway enrichment analysis of differential metabolites in CK and MJ
Larger bubbles indicate greater amount of differential metabolite enriched in the pathway; redder color indicates more significant enrichment of differential metabolite in the pathway.
表 1 MeJA对叶片悬浮细胞总酚、总黄酮、总三萜含量的影响
Table 1 Effects of MeJA on total phenol, flavonoid, and triterpene contents in leaf suspension cells
MeJA浓度
Concentration of MeJA / mg/L总酚含量
Phenol content / mg/g总黄酮含量
Flavonoid content / mg/g总三萜含量
Terpenoid content / mg/g0 54.60±1.08 240.07±32.53 133.45±12.29 0.125 53.05±1.69 284.23±29.26 161.11±15.42 0.25 63.10±1.36** 324.46±16.54* 166.90±4.57* 0.5 59.35±2.48* 277.01±12.29 147.61±1.71 1 63.31±1.59*** 330.00±8.41** 188.54±7.08** 2 79.41±1.48*** 380.77±0.65** 165.63±10.92* 4 69.59±1.43*** 415.46±4.03** 151.95±17.95 8 57.11±0.58* 245.64±19.39 110.19±9.39 Notes: *, P<0.05; **, P<0.01; ***, P<0.001. 表 2 CK和MJ的生物活性差异代谢物
Table 2 Bioactive differential metabolites of CK and MJ
类别
Category化合物名称
Compound name化学式
Chemical formula加合离子
AdductP值
P valueVIP值
VIP value差异倍数
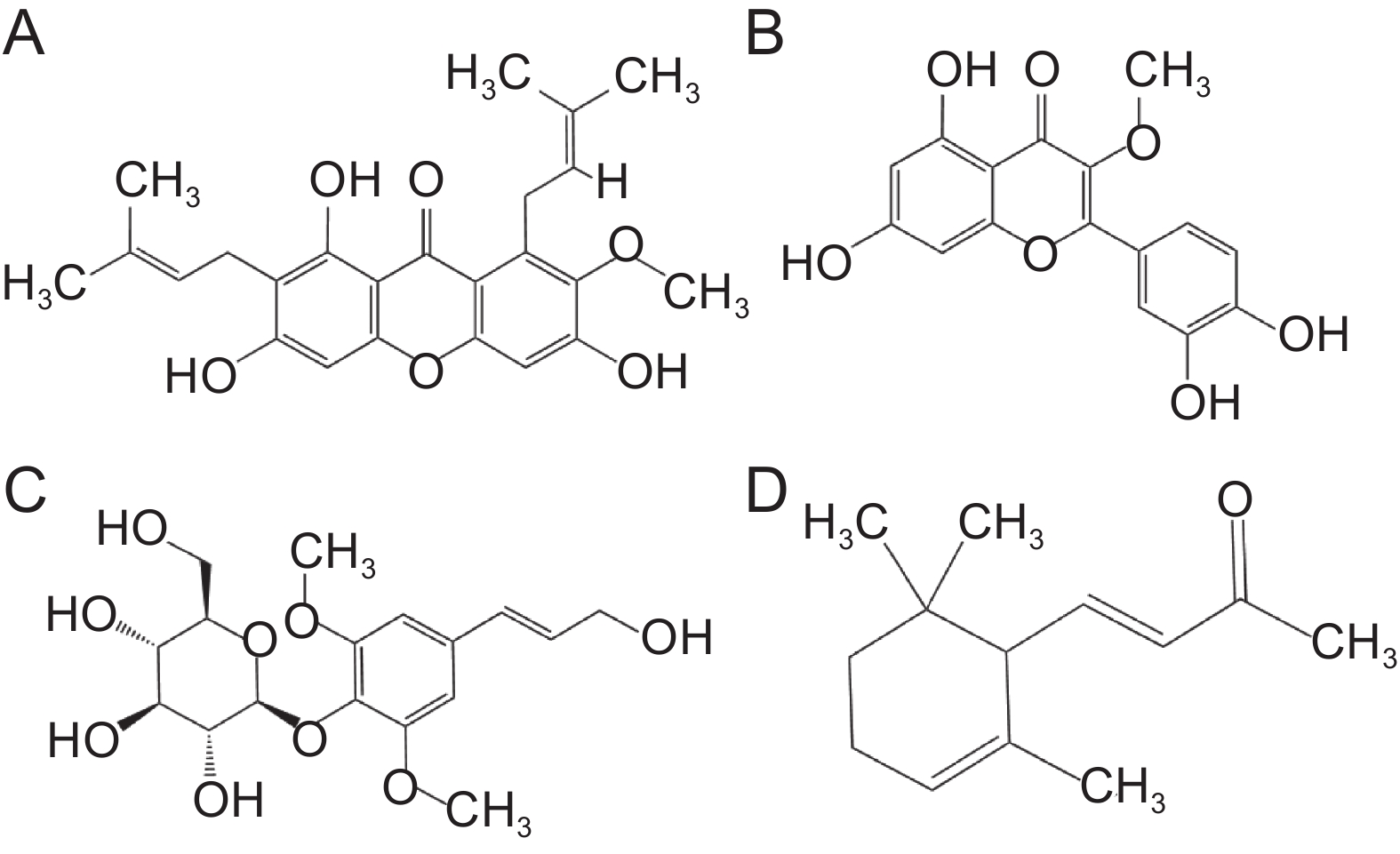
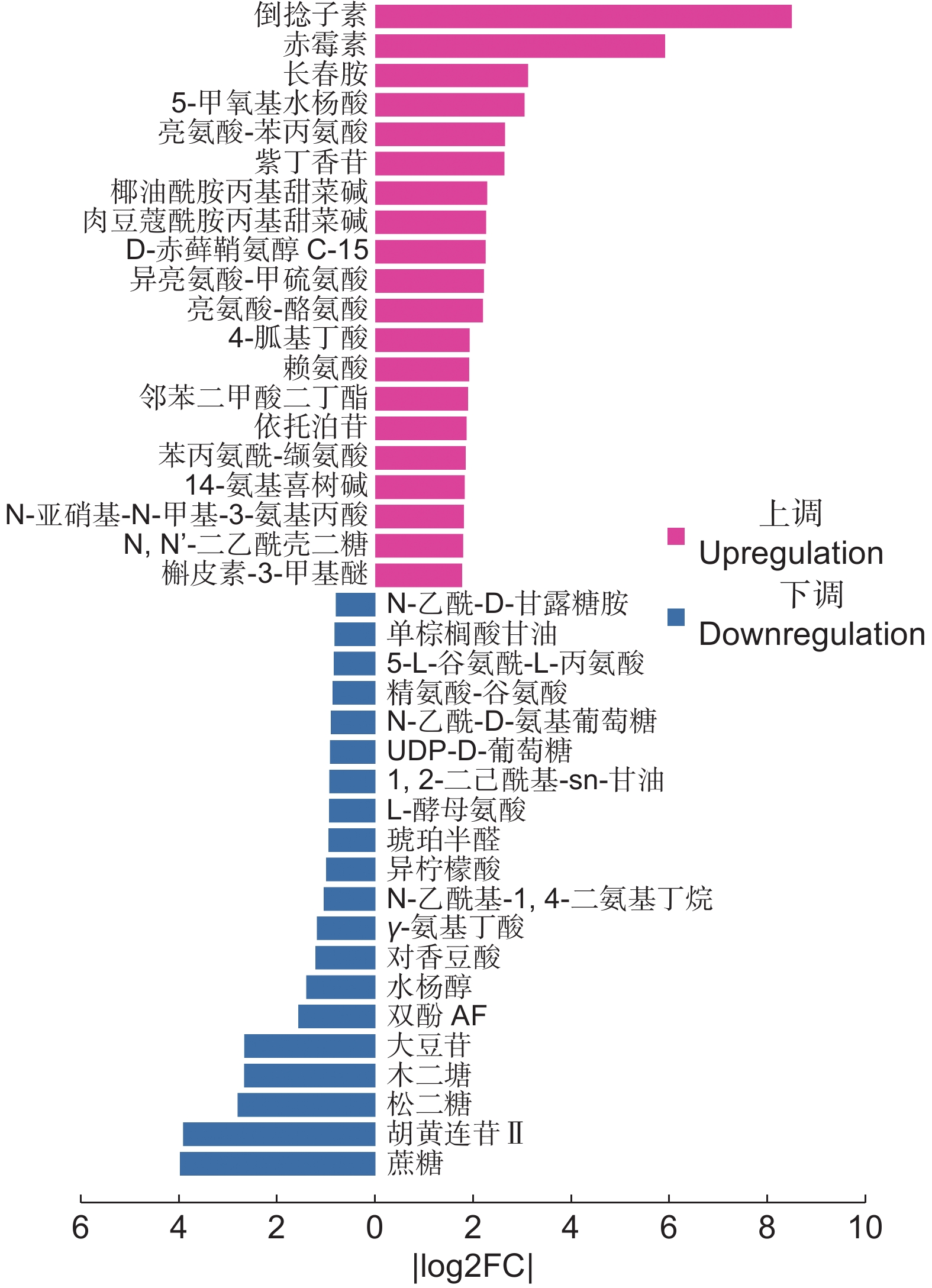
Fold change黄酮类 表儿茶素 C15H14O6 [M+H]+ 7.10×10−4 2.41 2.04 3,4,6-三甲氧基异黄酮-7-O-β-D-吡喃葡萄糖苷 C24H26O11 [M+Na]+ 9.54×10−4 1.11 1.74 丁香亭 C17H14O8 [M+H]+ 1.58×10−3 1.16 1.80 栀子黄素B C19H18O7 [M+H]+ 1.04×10−2 1.01 0.93 次野鸢尾黄素 C20H18O8 [M+Na]+ 1.06×10−5 1.65 2.47 根皮苷 C21H24O10 [M+H]+ 3.08×10−5 1.25 2.84 补骨脂定 C20H16O5 [M+H]+ 7.56×10−5 1.03 1.84 槲皮素-3-甲基醚 C17H14O7 [M+H]+ 1.31×10−4 1.69 2.97 大豆苷 C21H20O9 [M+Cl]− 6.64×10−3 1.28 0.16 酚酸类 倒捻子素 C24H26O6 [M+H]+ 4.62×10−7 4.01 359.35 蛇床子素 C15H16O3 [M+H]+ 2.49×10−3 1.19 2.52 胡黄连苷II C23H28O13 [M+H-H2O]+ 4.02×10−5 1.30 0.07 佛手酚 C11H6O4 [M+H]+ 7.26×10−4 5.38 1.50 紫丁香苷 C17H24O9 [M+Na]+ 1.48×10−3 1.28 4.85 依托泊苷 C29H32O13 [M+H]+ 4.96×10−3 1.14 3.57 阿魏酸 C10H10O4 [M-H]− 2.89×10−2 3.03 2.53 凯林 C14H12O5 [M+H]+ 6.88×10−5 1.09 2.09 状芸香素 C16H14O3 [M-H]− 5.07×10−5 1.25 1.56 对香豆酸 C9H8O3 [M-H-H2O]− 4.34×10−2 1.03 0.43 奎尼酸 C11H9NO3 [M-H]− 2.52×10−4 1.49 1.34 萜类 紫罗兰酮 C13H20O [M+H-H2O]+ 1.87×10−2 1.08 1.69 儿茶酚 C6H6O2 [M+H-3H2O]+ 6.89×10−4 1.47 2.18 球姜酮 C15H22O [M+H-H2O]+ 1.88×10−2 2.83 1.86 18β-甘草次酸 C30H46O4 [M-H]− 1.89×10−4 4.85 2.15 刺囊酸-3-O-葡萄糖苷 C36H58O9 [M-H]− 4.75×10−5 2.24 1.93 人参皂苷F3 C41H70O13 [M+FA-H]− 1.72×10−2 5.13 0.68 苜蓿酸+o-hex C35H54O11 [M-H]− 9.41×10−4 1.82 3.35 罗莎白素 C36H58O10 [M+Cl-]− 4.35×10−3 2.21 1.99 生物碱类 14-氨基喜树碱 C20H17N3O4 [M+H]+ 8.18×10−7 4.40 3.45 托品酮 C8H13NO [M+H]+ 1.43×10−2 2.24 2.46 长春胺 C21H26N2O3 [M+H]+ 4.03×10−6 1.29 8.23 醌类 恩贝灵 C17H26O4 [M-H]− 3.60×10−3 3.40 1.40 芪类 紫檀芪 C16H16O3 [M+H]+ 7.51×10−4 1.37 1.55 -
[1] Habán M,Korczyk-szabó J,Čerteková S,Ražná K. Lavandula species,their bioactive phytochemicals,and their biosynthetic regulation[J]. Int J Mol Sci,2023,24(10):8831. doi: 10.3390/ijms24108831
[2] Dobros N,Zawada KD,Paradowska K. Phytochemical profiling,antioxidant and anti-inflammatory activity of plants belonging to the Lavandula genus[J]. Molecules,2022,28(1):256. doi: 10.3390/molecules28010256
[3] Diass K,Merzouki M,Elfazazi K,Azzouzi H,Challioui A,et al. Essential oil of Lavandula officinalis:Chemical composition and antibacterial activities[J]. Plants (Basel),2023,12(7):1571. doi: 10.3390/plants12071571
[4] 陈日道,段瑞刚,邹建华,李军伟,刘晓月,等. 八角莲愈伤组织中黄酮苷类化学成分研究[J]. 中国中药杂志,2016,41(1):87−91. Chen RD,Duan RG,Zou JH,Li JW,Liu XY,et al. Flavonoid glycosides from callus cultures of Dysosma versipellis[J]. China Journal of Chinese Materia Medica,2016,41(1):87−91.
[5] Guerriero G,Berni R,Armando Muñoz-Sanchez J,Apone F,Abdel-Salam EM,et al. Production of plant secondary metabolites:examples,tips and suggestions for biotechnologists[J]. Genes (Basel),2018,9(6):309. doi: 10.3390/genes9060309
[6] 冯晓晖,闫学彤,郑珂媛,周强,张伟中,等. 富含紫杉烷类红豆杉的离体培养[J]. 植物学报,2023,58(6):917−925. doi: 10.11983/CBB22228 Feng XH,Yan XT,Zhen KY,Zhou Q,Zhang WZ,et al. In vitro culture of Taxus rich in taxanes[J]. Chinese Bulletin of Botany,2023,58(6):917−925. doi: 10.11983/CBB22228
[7] Xu F,Valappil AK,Mathiyalagan R,Tran TNA,Ramadhania ZM,et al. In vitro cultivation and ginsenosides accumulation in Panax ginseng:a review[J]. Plants (Basel),2023,12(17):3165. doi: 10.3390/plants12173165
[8] Luo C,Qiu JF,Zhang Y,Li MY,Liu P. Jasmonates coordinate secondary with primary metabolism[J]. Metabolites,2023,13(9):1008. doi: 10.3390/metabo13091008
[9] Serna-escolano V,Valverde JM,García-pastor ME,Valero D,Castillo S,et al. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters[J]. J Sci Food Agric,2019,99(11):5035−5043. doi: 10.1002/jsfa.9746
[10] Durand M,Besseau S,Papon N,Courdavault V. Unlocking plant bioactive pathways:omics data harnessing and machine learning assisting[J]. Curr Opin Biotechnol,2024,87:103135. doi: 10.1016/j.copbio.2024.103135
[11] Durmaz G. Freeze-dried ABTS+ method:a ready-to-use radical powder to assess antioxidant capacity of vegetable oils[J]. Food Chem,2012,133(4):1658−1663. doi: 10.1016/j.foodchem.2012.02.064
[12] Benzie IF,Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”:The FRAP assay[J]. Anal Biochem,1996,239(1):70−76. doi: 10.1006/abio.1996.0292
[13] 林月乔,张子桦,续文丽,李楠,刘辉,陈鑫. 莽吉柿果壳不同萃取物中总黄酮、总酚含量及抗菌活性[J]. 食品工业,2022,43(12):130−133. Lin YQ,Zhang ZH,Xu WL,Li N,Liu H,Chen X. Content of total flavonoids,phenols and its antibacterial activities of different extracts from Garcinia mangostana[J]. The Food Industry,2022,43(12):130−133.
[14] 黄梅桂,徐云巧,张忠明,应瑞峰,王耀松,王强. 薰衣草残渣中黄酮的超声辅助提取工艺及其抗氧化活性[J]. 食品工业科技,2018,39(1):214−220. Huang MG,Xu YQ,Zhang ZM,Ying RF,Wang YS,Wang Q. Flavonoids extracted by ultrasonic assisted method from lavender residue and its antioxidant activity[J]. Science and Technology of Food Industry,2018,39(1):214−220.
[15] 陈楠,吴潇霞,白冰瑶,陈计峦. 响应面法优化枣渣总三萜提取工艺及其抗氧化、抗增殖活性[J]. 食品研究与开发,2022,43(10):147−155. doi: 10.12161/j.issn.1005-6521.2022.10.020 Chen N,Wu XX,Bai BY,Chen JL. Optimization of extraction process of total triterpenoids from jujube residue by response surface methodology and the antioxidant and anti-proliferation activity[J]. Food Research and Development,2022,43(10):147−155. doi: 10.12161/j.issn.1005-6521.2022.10.020
[16] Nyamundanda G,Brennan L,Gormley IC. Probabilistic principal component analysis for metabolomic data[J]. BMC Bioinformatics,2010,11(1):571. doi: 10.1186/1471-2105-11-571
[17] Worley B,Powers R. Multivariate analysis in metabolomics[J]. Curr Metabolomics,2013,1(1):92−107.
[18] Wishart DS,Feunang YD,Marcu A,Guo AC,Liang K,et al. HMDB 4.0:the human metabolome database for 2018[J]. Nucleic Acids Res,2018,46(D1):D608−D617. doi: 10.1093/nar/gkx1089
[19] Kanehisa M,Furumichi M,Sato K,Kawashima M,Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes[J]. Nucleic Acids Res,2023,51(D1):D587−D592. doi: 10.1093/nar/gkac963
[20] Świątek A,Lenjou M,van Bockstaele D,Inzé D,van Onckelen HA. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells[J]. Plant Physiol,2002,128(1):201−211. doi: 10.1104/pp.010592
[21] Munteanu IG,Apetrei C. Analytical methods used in determining antioxidant activity:a review[J]. Int J Mol Sci,2021,22(7):3380. doi: 10.3390/ijms22073380
[22] Tjahjani S,Widowati W,Khiong K,Suhendra A,Tjokropranoto R. Antioxidant properties of Garcinia mangostana L. (mangosteen) rind[J]. Procedia Chemistry,2014(13):198−203.
[23] 杨东沛,张媛媛,叶火春,辜柳霜,冯岗,张静. α-倒捻子素对10种植物病原细菌的杀菌活性[J]. 热带农业科学,2023,43(6):45−50. Yang DP,Zhang YY,Ye HC,Gu LS,Feng G,Zhang J. Bactericidal activity of α-mangostin against ten kinds of plant pathogenic bacteria[J]. Chinese Journal of Tropical Agriculture,2023,43(6):45−50.
[24] 马林伟,徐红涛,戴小丽,韩中保. α-倒捻子素对MCF-7乳腺癌增殖、生长和侵袭的影响及其作用机制[J]. 中国现代医学杂志,2018,28(1):30−36. doi: 10.3969/j.issn.1005-8982.2018.01.007 Ma LW,Xu HT,Dai XL,Han ZB. Effect of α-mangostin on proliferation,growth and invasion of MCF-7 breast cancer and its mechanism[J]. China Journal of Modern Medicine,2018,28(1):30−36. doi: 10.3969/j.issn.1005-8982.2018.01.007
[25] Huang YQ,Cai SG,Ye LZ,Hu HL,Li CD,et al. The effects of GA and ABA treatments on metabolite profile of germinating barley[J]. Food Chem,2016(192):928−933.
[26] Dong YJ,Wu YX,Zhang ZX,Wang SC,Cheng J,et al. Transcriptomic analysis reveals GA3 is involved in regulating flavonoid metabolism in grape development for facility cultivation[J]. Mol Genet Genomics,2023,298(4):845−855. doi: 10.1007/s00438-023-02019-z
[27] Yao XH,Nie J,Bai RX,Sui XL. Amino acid transporters in plants:identification and function[J]. Plants (Basel),2020,9(8):972. doi: 10.3390/plants9080972
[28] Arruda P,Barreto P. Lysine catabolism through the saccharopine pathway:Enzymes and intermediates involved in plant responses to abiotic and biotic stress[J]. Front Plant Sci,2020,11:587. doi: 10.3389/fpls.2020.00587
[29] Galili G,Amir R,Fernie AR. The regulation of essential amino acid synthesis and accumulation in plants[J]. Annu Rev Plant Biol,2016,67:153−178. doi: 10.1146/annurev-arplant-043015-112213
[30] Yang QQ,Zhao DS,Zhang CQ,Wu HY,Li QF,et al. A connection between lysine and serotonin metabolism in rice endosperm[J]. Plant Physiol,2018,176(3):1965−1980. doi: 10.1104/pp.17.01283
[31] Forde BG,Lea PJ. Glutamate in plants:metabolism,regulation,and signalling[J]. J Exp Bot,2007,58(9):2339−2358. doi: 10.1093/jxb/erm121
[32] Van den Ende W,El-esawe SK. Sucrose signaling pathways leading to fructan and anthocyanin accumulation:a dual function in abiotic and biotic stress responses?[J]. Environ Exp Bot,2014,108:4−13. doi: 10.1016/j.envexpbot.2013.09.017
[33] Emanuelle S,Doblin MS,Stapleton DI,Bacic A,Gooley PR. Molecular insights into the enigmatic metabolic regulator,SnRK1[J]. Trends Plant Sci,2016,21(4):341−353. doi: 10.1016/j.tplants.2015.11.001
[34] Yu SM,Lo SF,Ho THD. Source-sink communication:regulated by hormone,nutrient,and stress cross-signaling[J]. Trends Plant Sci,2015,20(12):844−857. doi: 10.1016/j.tplants.2015.10.009
[35] Wurtzel ET,Kutchan TM. Plant metabolism,the diverse chemistry set of the future[J]. Science,2016,353(6305):1232−1236. doi: 10.1126/science.aad2062
[36] Do THT,Martinoia E,Lee Y,Hwang JU. 2021 update on ATP-binding cassette (ABC) transporters:how they meet the needs of plants[J]. Plant Physiol,2021,187(4):1876−1892. doi: 10.1093/plphys/kiab193
[37] Shitan N,Bazin I,Dan K,Obata K,Kigawa K,et al. Involvement of CjMDR1,a plant multidrug-resistance-type ATP-binding cassette protein,in alkaloid transport in Coptis japonica[J]. Proc Natl Acad Sci USA,2003,100(2):751−756. doi: 10.1073/pnas.0134257100
[38] Goodman CD,Casati P,Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays[J]. Plant Cell,2004,16(7):1812−1826. doi: 10.1105/tpc.022574
[39] Sagharyan M,Sharifi M,Samari E. Methyl jasmonate redirects the dynamics of carbohydrates and amino acids toward the lignans accumulation in Linum album cells[J]. Plant Physiol Biochem,2023,198:107677. doi: 10.1016/j.plaphy.2023.107677




 下载:
下载: