Research progress in plant Trehalose-6-phosphate synthase genes
-
摘要:
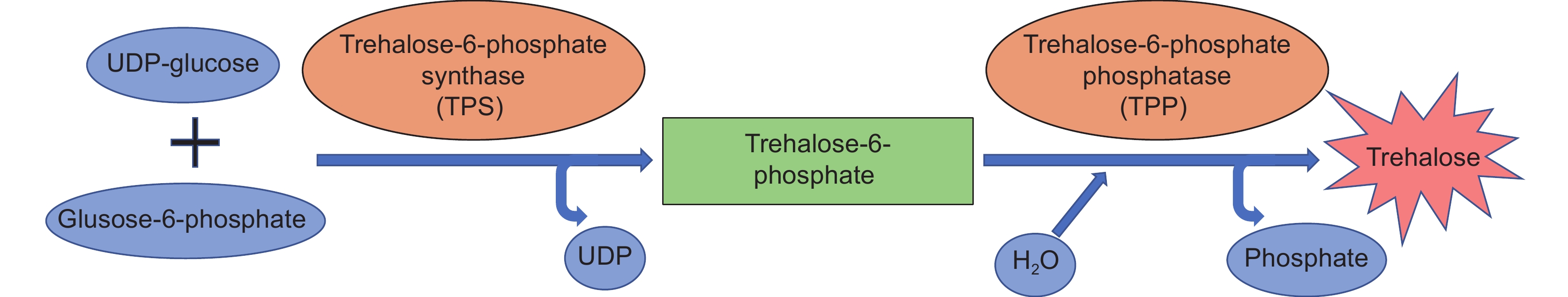
海藻糖-6-磷酸代谢通路是植物响应非生物胁迫生理调控网络中的重要组成部分,海藻糖-6-磷酸合成酶基因TPS(Trehalose-6-phosphate synthase)是植物合成海藻糖的关键基因。为了更加充分地理解TPS基因在植物应答非生物胁迫、调节开花时间等方面的作用,本文首先对植物TPS基因家族成员的系统进化关系及序列结构信息进行了研究,重点论述了TPS基因在植物响应干旱、盐害、高温、低温胁迫中的调控功能及分子作用机制,最后对TPS基因参与植物开花调控的研究进展进行了综述。本文深入总结了目前植物TPS基因响应非生物胁迫及开花调控的研究进展,并对今后TPS同源基因的研究方向和应用价值进行了展望。
-
关键词:
- 海藻糖-6-磷酸合成酶基因 /
- 非生物胁迫 /
- 开花调控
Abstract:The trehalose-6-phosphate metabolic pathway is an important component of the plant physiological regulation network in response to abiotic stress. Trehalose-6-phosphate synthase (TPS) is a key gene for trehalose synthesis in plants. To better understand the role of the TPS gene in plant response to abiotic stress and regulation of flowering time, this study presents an overview of current information on the phylogenetics and sequence structure of plant TPS gene family members, as well as the regulatory function and molecular mechanism of TPS in plant responses to drought, salt damage, high temperature, and low temperature stress, and research progress regarding its effects on plant flowering regulation. This study presents a comprehensive summary of research progress on the TPS gene in response to abiotic stress and flowering regulation, while also providing future research directions and potential applications of TPS homologs.
-
杜鹃为杜鹃花科杜鹃花属(Rhododendron)植物的统称,全世界有
1200 余种,我国有734种,其中464种为特有种[1-3]。杜鹃是我国传统十大名花之一,栽培历史悠久,具有较高的观赏、应用价值,且野生资源丰富。但相对于丰富的野生资源,国内杜鹃花种质创新进展较慢;迄今,欧美国家已登记的杜鹃花新品种数量高达几万种,我国近十年来共授权杜鹃花新品种163种[4]。目前杂交育种仍是杜鹃花属植物种质创新的主要技术手段[1, 4]。研究表明,杜鹃花亚属内杂交育种较容易,而亚属间杂交育种则难度较大,杂交亲和性较低,存在明显的生殖障碍,杂交成功率低[5-8]。但人们所期待的杜鹃花不同的表型性状,如不同花色、芳香、抗性等分散在不同亚属间,因此,克服亚属间杂交障碍是解决未来杜鹃花育种难点的钥匙[9]。开花生物学反映了植物的开花物候、开花进程、雌雄蕊发育以及雌雄配子发育特征,与植物繁衍息息相关[10, 11]。植物花期受植物自身生理调节的影响,同时光照、温度、坡向、降水量等环境因子也影响着开花物候[12-14]。花粉活力和柱头可授性是植物进行有性繁殖的基础,直接决定育种效率[15, 16]。花粉寿命以及柱头可授期的长短均与植物杂交育种密切相关[17, 18]。因此,深入研究杜鹃花属植物开花生物学特性,是其杂交选育的重要前提。映山红(R. simsii Planch.)属于映山红亚属(Subgen. Tsutsusi),是杜鹃花属中红色系花的理想亲本[19, 20];溪畔杜鹃(R. rivulare Hand.-Mazz.)也属于映山红亚属,花朵小巧雅致,花朵数量较多,花期长,观赏价值高[14];毛棉杜鹃(R. moulmainense Hook.)属于马银花亚属(Subgen. Azaleastrum),花大,适应性较好,且果实较大,是杂交育种中优良的父母本。前人已对锈叶杜鹃(R. siderophyllum Franch.)、云锦杜鹃(R. fortunei Lindl.)、多鳞杜鹃(R. polylepis Franch.)、大喇叭杜鹃(R. excellens Hemsl.)等的开花特性进行了研究[21-24],但针对这3种杜鹃生物学特性的系统研究尤其是在湖南地区的开花表现还未见报道。因此,本研究对两个亚属的3种杜鹃的开花生物学特性进行系统观测,探明其开花物候和开花进程及花粉活力和柱头可授性最强时期,以期为开展亚属内和亚属间杂交育种特性研究奠定基础。
1. 材料与方法
1.1 植物材料
实验地位于湖南省森林植物园国家林业草原杜鹃工程技术研究中心种质资源圃(28.10°N,113.03°E)。选择露地栽植、生长健壮且无病虫害的5年生映山红、溪畔杜鹃及毛棉杜鹃的实生苗作为研究材料。
1.2 开花动态及花器官特征观测
于2022年3-6月对3种杜鹃的群体和单花花期进行观测。每种杜鹃随机选择10~15株,记录其群体的始花期(25%个体开花)、盛花期(50%个体达到开花高峰时)、末花期(95%植株开花结束时)。每种杜鹃随机标记10~15朵单花,观测其开花动态。结合其开花特性,本研究将其单花开放进程划分为6个时期:松蕾期、露色期、喧蕾期、初开期、花冠平展期、花冠凋落期。采用游标卡尺测量不同时期花蕾/花筒长、花蕾/花冠直径、花梗长和直径等。
1.3 雄蕊发育形态观察及花粉活力的测定
于盛花期随机选取不同开放阶段的12~15朵发育良好的花蕾或花朵,采用游标卡尺测量其花药、花丝长度和直径等,于体视显微镜下观察花药和花丝形态及颜色变化、花药开裂情况,拍照并测量。同时另采集不同时期的15~20朵花,取下雄蕊,经2.5%戊二醛固定48 h后,采用邵凤侠等[25]的方法,对花药和花粉进行亚显微观测。
采用花粉离体培养法测定不同发育时期花粉活力。映山红和溪畔杜鹃的花粉采用预实验优选出的培养基(100 g/L蔗糖+10 mg/L H3BO3+300 mg/L CaCl2+1%琼脂)进行离体培养;毛棉杜鹃花粉采用白宇清等[26]筛选的最适培养基(200 g/L 蔗糖+100 mg/L H3BO3+300 mg/L CaCl2)进行离体培养;30 ℃培养10 h后于显微镜下观察。判断花粉萌发的依据是:四合体只要有一个萌发(即花粉管长度等于或超过花粉粒直径)则视为花粉已萌发,每个处理3次重复,每个重复选取5个视野计数,每个视野花粉粒不少于50粒。花粉萌发率(%)=已萌发花粉数/视野内花粉总数×100%。
1.4 柱头形态发育观察及可授性的测定
采集不同开放时期的花蕾或花朵25~30个,一部分于体视显微镜下观察花柱形态特征、颜色、柱头开裂情况,拍照并测量花柱和柱头直径(每个时期测量15个,取平均值);一部分剪取花柱,经2.5%戊二醛固定48 h后,采用邵凤侠等[27]的方法,对花柱和柱头进行亚显微观测。
采用联苯胺-过氧化氢法测定柱头可授性。按联苯胺乙醇溶液∶3%过氧化氢溶液∶水=4∶11∶22配制联苯胺-过氧化氢溶液。将各时期柱头放入培养皿,加入检测液直至淹没过柱头;反应8~10 min,于体视显微镜下观察并记录柱头表面气泡产生情况以及颜色变化。
1.5 数据分析
采用Excel 2019和SPSS 24.0软件进行数据整理和分析,采用Origin 2021软件作图。
2. 结果与分析
2.1 开花进程及花部形态特征
2.1.1 映山红
于2022年3-5月对映山红花期进行观测。映山红于3月中旬进入始花期,3月下旬进入盛花期,6~8 d后于4月初进入末花期,群体花期约20~25 d,单花花期11~15 d。花芽卵球形,总状花序2~6朵簇生枝顶(图1:A)。松蕾期:花苞被鳞片紧紧包裹;露色期:鳞片些许松动,花蕾顶部露出一点红色;喧蕾期:鳞片开始脱落,红色花蕾大部分露出,花蕾紧闭;初开期:鳞片完全脱落,花朵半开放,雄蕊开始散粉,花柱伸长稍高于花冠;花冠平展期:花朵完全开放,颜色鲜红,雄蕊完全散粉;花冠凋落期:花朵基部部分脱落并沿柱头方向下垂,雄蕊干瘪随花冠一起开始脱落,雌蕊宿存(图2:A1~F1)。随开放进程的推进,花蕾直径呈先增大而后缩减的趋势;蕾长和花梗长不断增长;花梗直径则无明显变化(图3)。
![]() 图 2 3种杜鹃不同开放时期A行为映山红;B行为溪畔杜鹃;C行为毛棉杜鹃。A:松蕾期;B:露色期;C:喧蕾期;D:初开期;E:花冠平展期;F:花冠凋落期。下同。标尺为1 cm。Figure 2. Three Rhododendron species at different opening stagesLine A is Rhododendron simsii; line B is Rhododendron rivulare; line C is Rhododendron moulmainense. A: Pine bud stage; B: Dewness stage; C: Bud stage; D: Ready-to-open period; E: Petal flat extension period; F: Corolla falling stage. Same below. Bar=1 cm.
图 2 3种杜鹃不同开放时期A行为映山红;B行为溪畔杜鹃;C行为毛棉杜鹃。A:松蕾期;B:露色期;C:喧蕾期;D:初开期;E:花冠平展期;F:花冠凋落期。下同。标尺为1 cm。Figure 2. Three Rhododendron species at different opening stagesLine A is Rhododendron simsii; line B is Rhododendron rivulare; line C is Rhododendron moulmainense. A: Pine bud stage; B: Dewness stage; C: Bud stage; D: Ready-to-open period; E: Petal flat extension period; F: Corolla falling stage. Same below. Bar=1 cm.2.1.2 溪畔杜鹃
于2022年4-6月对溪畔杜鹃花期进行观测。溪畔杜鹃从4月中旬进入始花期,4月下旬至5月初进入盛花期,8~10 d后在5月中旬进入末花期,群体花期约30 d;单花花期12~15 d。花芽圆锥状卵形,伞形花序10朵以上顶生(图1:B)。松蕾期:花序被鳞片包裹;露色期:鳞片些许松动,露出花蕾,顶部花瓣些许松动;喧蕾期:鳞片开始脱落,花蕾大部分露出,花瓣部分完全松动,雄蕊开始散粉;初开期:鳞片完全脱落,花冠基本开放,雌蕊伸长高于花冠;花冠平展期:花瓣平展,柱头分泌大量粘液,雄蕊完全散粉并开始干瘪;花冠凋落期:花冠、雄蕊凋落,柱头下垂,仅剩雌蕊(图2:A2~F2)。随开花进程的推进,花蕾直径和花蕾长呈先增大而后减弱的趋势;花梗长不断增长,花梗直径无明显变化(图3)。
2.1.3 毛棉杜鹃
于2022年4-5月对毛棉杜鹃花期进行观测。毛棉杜鹃从4月中旬进入始花期,4月下旬进入盛花期,3~5 d进入末花期,群体花期15~20 d;单花花期8~13 d。花芽长椭圆状卵形或长圆锥状卵形,数伞形花序生枝顶叶腋,每花序2~6朵花(图1:C)。松蕾期:鳞片紧紧包裹花序,花蕾稍高于鳞片1~2 cm,花蕾中部及以上呈深紫色,基部为淡粉色;露色期:鳞片松动,1/2花蕾露出,花蕾伸长,中部及以上呈紫色;喧蕾期:鳞片开始脱落,花蕾完全露出且开始膨大;初开期:鳞片完全脱落,花瓣开始展平但未完全展开,柱头有少量粘液分泌;花冠平展期:花冠完全绽放,雄蕊完全散粉,柱头分泌大量粘液;花冠凋落期:花冠萎蔫,花冠和花丝脱落,柱头下垂且边缘干枯(图2:A3~F3)。随开花进程的推进,花蕾直径、花蕾长和花梗长不断增长;花梗直径无明显变化(图3)。
2.2 雄蕊形态发育进程及花粉活力
2.2.1 不同开放时期雄蕊形态变化
3种杜鹃雄蕊形态和大小随开花进程呈规律性变化,但存在差异。其花药长度呈先增长而后缩短的变化,映山红花丝长不断增长,溪畔杜鹃花丝长则经历极速增长而后短暂下降再继续增长的过程,毛棉杜鹃花丝长则先快速增长而后逐渐平稳。在整个开花过程中,花药直径和花丝直径则无明显变化(图4)。映山红与毛棉杜鹃均为10枚雄蕊,溪畔杜鹃仅5枚雄蕊,花药顶端均有孔。3种杜鹃的花粉均为复合型四合体,呈正四面体,顶部1个花粉分体与基部3个花粉分体组合在一起;花粉表面粗糙,有颗粒状纹饰突起,具三裂萌发沟,3个萌发孔。花粉粒近球形或扁球形,具3个呈120°的孔沟,沟内内含物外露,花粉粒表面具较密集粘丝,将花粉粒缠绕在一起。
映山红不同开放时期雄蕊形态发育特征如图5所示。松蕾期:花药红色,花药壁表面光滑,细胞不规则;露色期:花药红色加深,花药壁表面光滑,细胞排列规则;喧蕾期:花药深红色,花药壁表面细胞排列规则紧凑;初开期:花药壁表面细胞排列规则紧凑,花粉开始从花药孔散出;花冠平展期:花药黑紫色、完全散粉,花药壁表面细胞数量增多排列规则紧凑,此时花药最大;花冠凋落期:花药黑紫色加深,开始干瘪,花药壁表面细胞开始塌陷、解体。花粉粒于松蕾期、露色期、喧蕾期以及花冠凋落期极面观近三角形,初开期极面观近长球形,至花冠平展期极面观近球形;花粉粒于松蕾期、露色期、花冠平展期赤面观近球形,于喧蕾期、初开期及花冠凋落期赤面观近椭圆形。从松蕾期到喧蕾期花粉极轴方向不断增长,即此阶段花粉一直在纵向增长;从初开期到花冠凋落期开始赤轴增长比极轴快,即花粉开始横向生长(附表1
1 ))。![]() 图 5 映山红单花不同开放时期花药及花粉的亚显微状态A-1~A-6:松蕾期;B-1~B-6:露色期;C-1~C-6:喧蕾期;D-1~D-6:初开期;E-1~E-6:花冠平展期;F-1~F-6:花冠凋落期。A-1和A-2、B-1和B-2、C-1和C-2、D-1和D-2、E-1和E-2、F-1和F-2均为花药;A-3和A-4、B-3和B-4、C-3和C-4、D-3和D-4、E-3和E-4、F-3和F-4均为花药表皮细胞;A-5和A-6、B-5和B-6、C-5和C-6、D-5和D-6、E-5和E-6、F-5和F-6均为花粉群体;A-7、B-7、C-7、D-7、E-7、F-7均为花粉。下同。Figure 5. Submicroscopic states of anthers and pollen of Rhododendron simsii at different opening stagesA-1 to A-6: Pine bud stage; B-1 to B-6: Dewness stage; C-1 to C-6: Bud stage; D-1 to D-6: Ready-to-open stage; E-1 to E-6: Petal flat extension stage; F-1 to F-6: Corolla falling stage. A-1 and A-2, B-1 and B-2, C-1 and C-2, D-1 and D-2, E-1 and E-2, F-1 and F-2: Anther; A-3 and A-4, B-3 and B-4, C-3 and C-4, D-3 and D-4, E-3 and E-4, F-3 and F-4: Anther epidermal cell; A-5 and A-6, B-5 and B-6, C-5 and C-6, D-5 and D-6, E-5 and E-6, F-5 and F-6: Pollen group; A-7, B-7, C-7, D-7, E-7, F-7: Pollen. Same below.
图 5 映山红单花不同开放时期花药及花粉的亚显微状态A-1~A-6:松蕾期;B-1~B-6:露色期;C-1~C-6:喧蕾期;D-1~D-6:初开期;E-1~E-6:花冠平展期;F-1~F-6:花冠凋落期。A-1和A-2、B-1和B-2、C-1和C-2、D-1和D-2、E-1和E-2、F-1和F-2均为花药;A-3和A-4、B-3和B-4、C-3和C-4、D-3和D-4、E-3和E-4、F-3和F-4均为花药表皮细胞;A-5和A-6、B-5和B-6、C-5和C-6、D-5和D-6、E-5和E-6、F-5和F-6均为花粉群体;A-7、B-7、C-7、D-7、E-7、F-7均为花粉。下同。Figure 5. Submicroscopic states of anthers and pollen of Rhododendron simsii at different opening stagesA-1 to A-6: Pine bud stage; B-1 to B-6: Dewness stage; C-1 to C-6: Bud stage; D-1 to D-6: Ready-to-open stage; E-1 to E-6: Petal flat extension stage; F-1 to F-6: Corolla falling stage. A-1 and A-2, B-1 and B-2, C-1 and C-2, D-1 and D-2, E-1 and E-2, F-1 and F-2: Anther; A-3 and A-4, B-3 and B-4, C-3 and C-4, D-3 and D-4, E-3 and E-4, F-3 and F-4: Anther epidermal cell; A-5 and A-6, B-5 and B-6, C-5 and C-6, D-5 and D-6, E-5 and E-6, F-5 and F-6: Pollen group; A-7, B-7, C-7, D-7, E-7, F-7: Pollen. Same below.溪畔杜鹃不同开放时期雄蕊形态发育特征如图6所示。松蕾期:花药紫红色,花药壁光滑,表面细胞发育不完全但已具细胞形态;露色期:花药紫红色,花药壁凹凸不平,表面细胞部分开始向上膨大、有褶皱;喧蕾期:花药颜色加深,表面细胞边缘褶皱消失,呈不规则长椭圆状紧密排列,花药孔开始散粉;初开期:花药紫黑色,表面细胞发育完全;花冠平展期:花药暗紫红色,完全散粉,表面细胞部分开始皱缩;花冠凋落期:花药褐色,花药壁完全皱缩,表面细胞塌陷。花粉粒于松蕾期、露色期、初开期极面观近扁球形,花冠凋落期极面观近球形,至花冠平展期、花冠凋落期极面观又呈三角形;初开期、花冠凋落期赤面观近三角形,露色期、初开期赤面观近扁球形,而松蕾期、花冠平展期赤面观均呈近圆形。花粉粒从松蕾期到初开期一直沿赤轴方向增长,但从初开期到花冠凋落期开始,极轴增长也逐渐加快(附表1)。
毛棉杜鹃不同开放时期雄蕊形态发育特征如图7所示。松蕾期:花药淡紫色,表面细胞排列成不规则小斑块,有明显褶皱;露色期:花药紫色,表面细胞开始伸长,大部分细胞向上膨起;喧蕾期:花药颜色变淡,呈淡紫色,开始散粉,表面细胞继续伸长,排成不规则长矩形;初开期:花药粉紫色,表面细胞发育完全,花粉散出;花冠平展期:花药部分变褐,表面细胞边缘开始凹陷坍塌,完全散粉;花冠凋落期:花药褐色,表面细胞完全凹陷衰老。花粉粒于松蕾期、露色期、喧蕾期、花冠凋落期四个时期极面观均近三角形,初开期极面观为扁圆形,至花冠平展期花粉粒极面观近圆形;花粉在松蕾期、露色期、喧蕾期、初开期花粉粒的赤面观均为三角形,而在花冠平展期、花冠凋落期赤面观呈扁圆形。花粉粒从松蕾期到喧蕾期缓慢沿着极轴方向增长;从初开期开始直到花冠凋落期就一直沿赤轴方向增长(附表1)。
2.2.2 不同开放时期花粉活力
3种杜鹃花粉活力随开花进程均呈先升后降的趋势。松蕾期花粉均无活力或活力极低,从露色期开始花粉均具备活力,且该时期溪畔杜鹃花粉活力显著高于其他两种。映山红花冠平展期花粉活力最强(82.77%),溪畔杜鹃喧蕾期花粉活力最强(55.66%),毛棉杜鹃则在初开期花粉活力最强(81.86%)。3种杜鹃花粉活力从花冠平展期呈下降趋势,随雄蕊衰败凋落,花粉逐渐失活。松蕾期和初开期时毛棉杜鹃花粉活力与其他两种杜鹃差异显著,溪畔杜鹃花粉活力在露色期和花冠平展期时与其他两种有显著差异,喧蕾期及凋落期3种杜鹃花粉活力均存在显著差异(图8)。
![]() 图 8 3种杜鹃不同开放时期花粉活力不同大写字母表示不同杜鹃同一开放时期差异显著(P<0.05);不同小写字母表示同种杜鹃不同开放时期差异显著(P<0.05)。Figure 8. Pollen activity of three Rhododendron species at different opening stagesDifferent capital letters indicate difference in the same opening stage of different varieties (P<0.05); Different lowercase letters indicate significant difference in different opening stages of the same variety (P<0.05).
图 8 3种杜鹃不同开放时期花粉活力不同大写字母表示不同杜鹃同一开放时期差异显著(P<0.05);不同小写字母表示同种杜鹃不同开放时期差异显著(P<0.05)。Figure 8. Pollen activity of three Rhododendron species at different opening stagesDifferent capital letters indicate difference in the same opening stage of different varieties (P<0.05); Different lowercase letters indicate significant difference in different opening stages of the same variety (P<0.05).2.3 雌蕊形态发育进程及柱头可授性
2.3.1 不同开放时期花柱形态特征
3种杜鹃柱头五裂,均有粘液分泌,为湿性柱头。花柱和柱头大小随着开花进程整体上呈先增长后减弱趋势。毛棉杜鹃增长较为平缓,而映山红和溪畔杜鹃在初开期与花冠平展期两个时期之间有明显增长,而后也逐渐变得平缓。3种杜鹃花柱直径从喧蕾期至花冠凋落期均无明显变化(图9)。
映山红不同开放时期花柱形态特征见图10。松蕾期:花柱绿色,表面细胞呈不规则圆形排列、有竖条纹路;柱头乳突细胞未发育完全,不具细胞形态,干瘪皱缩。露色期:花柱中部及以上变成橙红色,柱头仍为绿色;花柱表面细胞呈长椭圆形、纹路加深;柱头乳突细胞中间部分开始向上膨大,初具细胞形态。喧蕾期:花柱完全红色,表面纹路增多并向外凸起;柱头稍带红色、有少量粘液分泌,乳突细胞已具细胞形态、排列成斑块状向上凸起。初开期:花柱和柱头完全变红;花柱表面细胞纹路横向条纹数量增多;柱头表面粘液增多,具一层蛋白质薄膜,乳突细胞继续向上膨大、排列松散。花冠平展期:花柱和柱头颜色加深,柱头表面有大量粘液,乳突细胞发育完全。花冠凋落期:花柱和柱头黑红色;柱头表面变干,无粘液分布或有少量粘液分布,乳突细胞解体。
![]() 图 10 映山红单花不同开放阶段柱头与花柱亚显微状态A-1~A-7:松蕾期;B-1~B-7:露色期;C-1~C-7:喧蕾期;D-1~D-7:初开期;E-1~E-7:花冠平展期;F-1~F-7:花冠凋落期。A-1和A-2、B-1和B-2、C-1和C-2、D-1和D-2、E-1和E-2、F-1和F-2均为柱头;A-3和A-4、B-3和B-4、C-3和C-4、D-3和D-4、E-3和E-4、F-3和F-4均为花柱表皮细胞;A-5、B-5、C-5、D-5、E-5、F-5均为柱头表面;A-6和A-7、B-6和B-7、C-6和C-7、D-6和D-7、E-6和E-7、F-6和F-7均为柱头乳突细胞;下同。Figure 10. Submicroscopic state of style and stigma of Rhododendron simsii at different opening stagesA-1 to A-7: Pine bud stage; B-1 to B-7: Dewness stage; C-1 to C-7: Bud stage; D-1 to D-7: Ready-to-open stage; E-1 to E-7: Petal flat extension stage; F-1 to F-7: Corolla falling stage. A-1 and A-2, B-1 and B-2, C-1 and C-2, D-1 and D-2, E-1 and E-2, F-1 and F-2: Stigma; A-3 and A-4, B-3 and B-4, C-3 and C-4, D-3 and D-4, E-3 and E-4, F-3 and F-4: Style epidermal cell; A-5, B-5, C-5, D-5, E-5, F-5: Stigma surface; A-6 and A-7, B-6 and B-7, C-6 and C-7, D-6 and D-7, E-6 and E-7, F-6 and F-7: Stigma papilla cell. Same below.
图 10 映山红单花不同开放阶段柱头与花柱亚显微状态A-1~A-7:松蕾期;B-1~B-7:露色期;C-1~C-7:喧蕾期;D-1~D-7:初开期;E-1~E-7:花冠平展期;F-1~F-7:花冠凋落期。A-1和A-2、B-1和B-2、C-1和C-2、D-1和D-2、E-1和E-2、F-1和F-2均为柱头;A-3和A-4、B-3和B-4、C-3和C-4、D-3和D-4、E-3和E-4、F-3和F-4均为花柱表皮细胞;A-5、B-5、C-5、D-5、E-5、F-5均为柱头表面;A-6和A-7、B-6和B-7、C-6和C-7、D-6和D-7、E-6和E-7、F-6和F-7均为柱头乳突细胞;下同。Figure 10. Submicroscopic state of style and stigma of Rhododendron simsii at different opening stagesA-1 to A-7: Pine bud stage; B-1 to B-7: Dewness stage; C-1 to C-7: Bud stage; D-1 to D-7: Ready-to-open stage; E-1 to E-7: Petal flat extension stage; F-1 to F-7: Corolla falling stage. A-1 and A-2, B-1 and B-2, C-1 and C-2, D-1 and D-2, E-1 and E-2, F-1 and F-2: Stigma; A-3 and A-4, B-3 and B-4, C-3 and C-4, D-3 and D-4, E-3 and E-4, F-3 and F-4: Style epidermal cell; A-5, B-5, C-5, D-5, E-5, F-5: Stigma surface; A-6 and A-7, B-6 and B-7, C-6 and C-7, D-6 and D-7, E-6 and E-7, F-6 and F-7: Stigma papilla cell. Same below.溪畔杜鹃不同开放时期花柱形态特征见图11。松蕾期:花柱表面细胞椭圆状;柱头呈纺锤状,乳突细胞干瘪皱缩。露色期:花柱伸长;柱头半椭圆形,表面有一层蛋白质薄膜,乳突细胞向上凸起。喧蕾期:花柱表面细胞继续向外凸起、出现不规则条纹;柱头向内收缩,表面蛋白质薄膜分散到每一裂片上,乳突细胞继续膨大。初开期:花柱外层细胞粘合成丝带状组织;柱头呈半球状,表面蛋白质薄膜开始裂解,有少量球体状蛋白质粘在薄膜碎片上,乳突细胞向上伸长,排列不均匀。花冠平展期:花柱外层细胞开始皱缩;柱头表面有明显粘液沉淀,表面蛋白膜大部分解体,且有许多蛋白球分布,乳突细胞呈椭圆状。花冠凋落期:花柱外层细胞已不具细胞形态;柱头萎蔫,边缘干枯,表面粘液明显减少但仍有大量颗粒状蛋白粘附,乳突细胞萎缩干瘪。
毛棉杜鹃不同开放时期花柱形态特征见图12。松蕾期:花柱外层细胞呈不规则块状分布;柱头发育不完全,乳突细胞干瘪。露色期:花柱外层细胞生长逐渐规则;柱头向上凸起,乳突细胞向上凸起并伸长成各种不规则形状。喧蕾期:花柱外层细胞表面出现竖条纹;表面有少量粘液附着。初开期:花柱表面细胞排成长矩形;柱头分泌大量粘液,乳突细胞继续伸长、向上凸起。花冠平展期:花柱表面细胞开始皱缩;柱头表面有明显的粘液沉积,边缘逐渐干硬,乳突细胞发育完全且边缘开始坍塌。花冠凋落期:花柱表面细胞完全塌陷;柱头萎蔫,乳突细胞解体。
2.3.2 不同开放时期柱头可授性
3种杜鹃柱头可授性检测结果见表1。不同开放时期柱头可授性存在差异,整体上呈现先增强后减弱趋势。映山红和毛棉杜鹃在花冠平展期可授性最强,溪畔杜鹃则在初开期就具有强可授性。3种杜鹃均在露色期具有可授性,柱头形态、颜色和柱头表面乳突组织生长发育程度与可授性紧密相关:柱头可授性最强时期柱头颜色均相应加深,柱头表面有大量粘液附着或沉积,乳突细胞发育完全、形态饱满。
表 1 不同开放阶段柱头可授性Table 1. Stigma receptivity at different opening stages时期
Stage映山红
Rhododendron simsii溪畔杜鹃
Rhododendron rivulare毛棉杜鹃
Rhododendron moulmainense柱头形态特征
Morphological characteristics of stigma柱头可授性
Stigma receptivity柱头形态特征
Morphological characteristics of stigma柱头可授性
Stigma receptivity柱头形态特征
Morphological characteristics
of stigma柱头可授性
Stigma receptivity松蕾期 纺锤状,绿色;乳突细胞未发育完全 +/– 呈纺锤状,深绿色;乳突细胞干瘪皱缩 +/– 纺锤状,嫩绿色;乳突细胞干瘪 +/– 露色期 绿色,部分开始向上凸起;乳突细胞中间部分开始向上膨大 + 颜色略变浅,柱头成半椭圆形,开始分泌粘液;乳突细胞向上凸起 + 深绿色,柱头部分向上凸起;乳突细胞也向上凸起并伸长成各种不规则形状 + 喧蕾期 稍带红色,已有少量粘液分泌;乳突细胞成斑块状向上凸起 ++ 淡绿色,向内收缩;乳突细胞继续膨大 ++ 有少量粘液附着,柱头浅绿色;乳突细胞继续伸长膨大 ++ 初开期 红色,表面粘液分泌增多,形成一层蛋白质薄膜;乳突细胞继续向上膨大 ++ 部分呈半球状,黄绿色,分泌大量粘液;乳突细胞向上伸长 +++ 继续向上凸起,分泌大量粘液,黄绿色;乳突细胞继续向上凸起 ++ 花冠平展期 深红色,大量粘液分布;乳突细胞发育完全 +++ 黄绿色,每一裂表面逐渐平宽,有明显粘液沉淀;乳突细胞呈椭圆状 +++ 表面有明显粘液沉积,柱头米黄色,边缘逐渐干硬;乳突细胞发育完全 +++ 花冠凋落期 表面开始变干,基本无粘液或有少量粘液分布;乳突细胞解体 + 萎蔫,边缘干枯,黄白色,粘液减少;乳突细胞萎缩干瘪 + 萎蔫变褐,表面干枯;乳突细胞解体 + 注:“–”表示柱头不具可授性;“+/–”指部分柱头具可授性;“+”表示柱头具可授性;“++ ”表示柱头可授性较强;“+++”表示可授性最强。 Notes: “–” means stigma has no receptivity; “+/–” means part of stigma has receptivity; “+” means stigma has receptivity; “++ ” means stigma has strong receptivity; “+++” means stigma has the highest receptivity. 3. 讨论
3.1 花期及花部形态特征
本研究发现,3种杜鹃花期不同,这与已报道的其他杜鹃花期也有所不同,如锈叶杜鹃[21]花期2-7月,多鳞杜鹃[23]花期4月上旬至5月初,云锦杜鹃[22]和大喇叭杜鹃[28]均于5月上中旬开花,波叶杜鹃[29]与大叶金顶杜鹃(R. faberisp Subsp. prattii (Franch.) Chamb.)[30]花期5月初至6月中旬,长梗杜鹃(R. longipedicellatum L. Cai & Y. P. Ma)[31]花期12月中旬至1月中旬。花期同步对提高杂交育种效率至关重要,若对这3种杜鹃开展杂交育种工作,需考虑三者之间花期不遇的情况以及与其他杜鹃花期不遇的情况。另外花朵形态特征也影响育种效率,花期持续时间、开花数与座果数均呈显著正相关;开花数多有利于吸引更多传粉者,花期长则有利于提高传粉效率,进而提高座果数[23]。本研究中溪畔杜鹃单个花蕾内花朵数显著多于映山红和毛棉杜鹃,花朵小而密集,雄蕊花粉量较少且活性较低,柱头小且远高于花冠;空间上,雌雄蕊存在明显异位。但溪畔杜鹃较其他两种杜鹃花期较长,可在较长时间内完成授粉受精,也为其人工育种提供了较充足的时间。
3.2 雄蕊形态发育特征
3种杜鹃雄蕊数不同,映山红和毛棉杜鹃雄蕊数均为10,而溪畔杜鹃仅有5枚雄蕊,均不等长。随着开花进程,花药颜色均逐渐加深。3种杜鹃花药顶端有孔,均以孔裂的方式进行散粉;映山红初开期开始散粉,溪畔杜鹃和毛棉杜鹃均于喧蕾期开始散粉。花粉粒一般都有粘性或者特殊纹饰,便于粘附在传粉者口部、腿部或者身体其他部位[28]。植物会针对不同传粉者产生不同的花粉连接线,这提高了植物自身的繁殖率[31]。花粉表面均有大量粘丝,散粉时呈串丝状散出,极大提高了花粉的输出效率,也可提高人工授粉的成功率。3种杜鹃花粉活力存在显著差异,活力较高时期均在初开期和花冠平展期,但都低于大喇叭杜鹃(84.43%)[28]、波叶杜鹃(91.05%)[29]、大叶金顶杜鹃(95%以上)[30]和锦绣杜鹃(88.98%)[32]。其中,映山红和毛棉杜鹃花粉活力高于常绿杜鹃品种“ XXL ”(80.75%)[33]。白宇清[34]对毛棉杜鹃开花前两天和当天的花粉活力测定结果显示,其平均值均在95%以上,远高于本研究结果,造成这种差异的原因可能在于本研究培养温度过高导致部分花粉失活。不同植物在同一环境下花粉活力与寿命有差异[30],映山红和毛棉杜鹃花粉活力从初开期到花冠凋落期都保持50%以上,而溪畔杜鹃花粉活力仅在喧蕾期和初开期才达到50%以上;同时在研究过程中发现阴雨天气时,3种杜鹃的散粉量远低于天气晴朗时,但阴雨天是否会导致其花粉活力下降还需进一步验证。远距离杂交和花期不遇是杂交育种中最常见的问题,花粉活力是人工授粉成功的关键[24, 35],因此通过花粉贮藏保持花粉活力尤为重要,但3种杜鹃花粉贮藏最适合条件还需进一步探究。
3.3 雌蕊形态发育特征
柱头是接受花粉的部位,表型特征影响着传粉效率,湿柱头表面分泌物可以为花粉萌发提供条件[36]。3种杜鹃的柱头均为湿柱头,其形态随开花进程发生变化:映山红柱头形态呈“扁圆-椭圆”变化,颜色呈“绿色-红绿色-橘红色-红色-深红色”变化;溪畔杜鹃柱头形态变化过程为“椭圆-扁圆-椭圆-扁圆”,颜色呈“深绿色-浅绿-黄绿-黄褐色-米黄”变化;毛棉杜鹃柱头形态呈“椭圆-扁圆-椭圆”变化,颜色变化为“深绿色-浅绿-米黄-灰褐”。柱头可授性强弱影响着杂交育种效率,映山红和毛棉杜鹃柱头可授性最强时期为花冠平展期,而溪畔杜鹃柱头于初开期就具有强可授性;映山红柱头可授性最强时期仅1~2 d,溪畔杜鹃和毛棉杜鹃柱头可授期稍长,为2~3 d,这与白宇清[34]的研究结果一致。3种杜鹃从松蕾期到初开期柱头形态逐渐饱满圆润,柱头表面粘液分泌增多,表面乳突细胞也从干瘪皱缩到饱满,此期间柱头可授性不断增强;初开期到花冠凋落期柱头也逐渐萎焉,粘液减少直至最后干枯,表面乳突细胞又从饱满到皱缩解体,柱头可授性也随之减弱,这与枣花(Ziziphus jujuba Mill.)柱头[27]和月季(Rosa hybrida E.H.L.Krause)柱头[37]的研究结果相似。柱头可授性与表面乳突细胞的发育程度密切相关,因此可依据植物柱头表面乳突组织发育程度判断其可授性的强弱[27]。
综上可知,3种杜鹃花期不遇且都存在雌雄异熟现象,均是雄蕊先熟,雌蕊后熟,使其自交比较困难,自然条件下结实率低,可人工辅助授粉。3种杜鹃花粉在露色期就已具有活力,因此若以这3种杜鹃作为杂交母本需把握去雄时间避免柱头因自交而污染;而将其作为杂交父本时可根据本研究结果把握花粉采集时间。在将来3种杜鹃杂交育种工作中,可根据雌蕊形态发育特征判断其最佳授粉时期,有效地提高杂交育种效率。
-
表 1 植物TPS基因家族的鉴定和功能验证情况
Table 1 Identification and functional verification of TPS gene family in plants
植物种类
Plant speciesTPS基因成员数
No. of TPS family members转入基因
Transferred genes启动子
Promoter转化植物
Transformed plant主要效应
Main effects文献
Reference苹果 Malus pumila Mill. 13 无 无 无 无 [10] 白杨 Populus tomentosa Carr. 13 无 无 无 无 [25] 荷花 Nelumbo nucifera Gaertn. 9 无 无 无 无 [27] 马铃薯 Solanum tuberosum L. 8 无 无 无 无 [28] 甘蔗 Saccharum officinarum L. 9 无 无 无 无 [29] 黄瓜 Cucumis sativus L. 7 无 无 无 无 [30] 大麻 Cannabis sativa L. 10 无 无 无 无 [32] 葡萄 Vitis vinifera L. 11 无 无 无 无 [34] 梅 Prunus mume Siebold & Zucc. 9 无 无 无 无 [35] 水稻 Oryza sativa L. 11 OsTPS1 Actin 1 水稻 提高了植株对低温、高盐、干旱等胁迫的耐受性 [37] 木薯 Manihot esculenta Crantz 12 MeTPS1 Ca MV35S 烟草 根茎叶中海藻糖积累增加,对干旱的耐受性增强 [38] 蒺藜苜蓿 Medicago truncatula L. 11 无 无 无 无 [42] 拟南芥 Arabidopsis thaliana (L.) Heynh. 11 AtTPS1 Ca MV35S 烟草 对干旱和高、低温的耐受性增强 [43] AtTPS1 35S 拟南芥 更耐脱水,抗性提高,对葡萄糖和脱落酸不敏感 [54] 番薯 Ipomoea batatas (L.) Lam. 1 IbTPS1 Ca MV35S 烟草 海藻糖和脯氨酸含量增加,耐盐性增强 [45] 西瓜 Citrullus lanatus (Thunb.) Matsum. & Nakai 7 ClTPS3 Ca MV35S 拟南芥 耐盐性显著增强 [46] 番茄 Solanum lycopersicum L. 11 无 无 无 无 [49] 大白菜 Brassica campestris L. 15 无 无 无 无 [50] 小麦 Triticum aestivum L. 12 TaTPS11 Ca MV35S 拟南芥 抗寒性增强 [53] 月季 Rosa chinensis Jacq. 9 无 无 无 无 [55] 新铁炮百合 Lilium × formolongi I. 9 LfTPS1/3/5 Ca MV35S 拟南芥 开花提前 [56] 辣木 Moringa oleifera Lam. 8 MoTPS1/2 无 拟南芥 开花提前 [57] 小桐子 Jatropha curcas L. 1 JcTPS1 Ca MV35S 拟南芥 开花提前 [58] -
[1] Lunn JE,Delorge I,Figueroa CM,Van Dijck P,Stitt M. Trehalose metabolism in plants[J]. Plant J,2014,79 (4):544−567. doi: 10.1111/tpj.12509
[2] Acosta-Pérez P,Camacho-Zamora BD,Espinoza-Sánchez EA,Gutiérrez-Soto G,Zavala-García F,et al. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase genes and analysis of its differential expression in maize (Zea mays) seedlings under drought stress[J]. Plants,2020,9 (3):315. doi: 10.3390/plants9030315
[3] Yatsyshyn VY,Kvasko AY,Yemets AI. Genetic approaches in research on the role of trehalose in plants[J]. Cytol Genet,2017,51 (5):371−383. doi: 10.3103/S0095452717050127
[4] Tang B,Wang S,Wang SG,Wang HJ,Zhang JY,Cui SY. Invertebrate trehalose-6-phosphate synthase gene:genetic architecture,biochemistry,physiological function,and potential applications[J]. Front Physiol,2018,9:30. doi: 10.3389/fphys.2018.00030
[5] Wang PL,Lei XJ,Lü JX,Gao CQ. Overexpression of the ThTPS gene enhanced salt and osmotic stress tolerance in Tamarix hispida[J]. J For Res,2022,33 (1):299−308. doi: 10.1007/s11676-020-01224-5
[6] Ma C,Wang ZQ,Kong BB,Lin TB. Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery[J]. Plant Growth Regul,2013,70 (3):275−285. doi: 10.1007/s10725-013-9799-2
[7] Vandesteene L,López-Galvis L,Vanneste K,Feil R,Maere S,et al. Expansive evolution of the TREHALOSE-6-PHOSPHATE PHOSPHATASE gene family in Arabidopsis[J]. Plant Physiol,2012,160 (2):884−896. doi: 10.1104/pp.112.201400
[8] Fichtner F,Lunn JE. The role of trehalose 6-phosphate (Tre6P) in plant metabolism and development[J]. Annu Rev Plant Biol,2021,72:737−760. doi: 10.1146/annurev-arplant-050718-095929
[9] Wahl V,Ponnu J,Schlereth A,Arrivault S,Langenecker T,et al. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana[J]. Science,2013,339 (6120):704−707. doi: 10.1126/science.1230406
[10] Du LS,Qi SY,Ma JJ,Xing LB,Fan S,et al. Identification of TPS family members in apple (Malus x domestica Borkh.) and the effect of sucrose sprays on TPS expression and floral induction[J]. Plant Physiol Biochem,2017,120:10−23. doi: 10.1016/j.plaphy.2017.09.015
[11] De Virgilio C,Bürckert N,Bell W,Jeno P,Boller T,Wiemken A. Disruption of TPS2,the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae,causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity[J]. Eur J Biochem,1993,212 (2):315−323. doi: 10.1111/j.1432-1033.1993.tb17664.x
[12] Kaasen I,Falkenberg P,Styrvold OB,Strøm AR. Molecular cloning and physical mapping of the otsBA genes,which encode the osmoregulatory trehalose pathway of Escherichia coli:evidence that transcription is activated by katF (AppR)[J]. J Bacteriol,1992,174 (3):889−898. doi: 10.1128/jb.174.3.889-898.1992
[13] Kaasen I,McDougall J,Strøm AR. Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase/phosphatase complex[J]. Gene,1994,145 (1):9−15. doi: 10.1016/0378-1119(94)90316-6
[14] Blázquez MA,Santos E,Flores CL,Martinez-Zapater JM,Salinas J,Gancedo C. Isolation and molecular characterization of the Arabidopsis TPS1 gene,encoding trehalose-6-phosphate synthase[J]. Plant J,1998,13 (5):685−689. doi: 10.1046/j.1365-313X.1998.00063.x
[15] Delorge I,Janiak M,Carpentier S,van Dijck P. Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants[J]. Front Plant Sci,2014,5:147.
[16] Delorge I,Figueroa CM,Feil R,Lunn JE,van Dijick P. Trehalose-6-phosphate synthase 1 is not the only active TPS in Arabidopsis thaliana[J]. Biochem J,2015,466 (2):283−290. doi: 10.1042/BJ20141322
[17] Lunn JE. Gene families and evolution of trehalose metabolism in plants[J]. Funct Plant Biol,2007,34 (6):550−563. doi: 10.1071/FP06315
[18] Ramon M,De Smet I,Vandesteene L,Naudts M,Leyman B,et al. Extensive expression regulation and lack of heterologous enzymatic activity of the Class Ⅱ trehalose metabolism proteins from Arabidopsis thaliana[J]. Plant Cell Environ,2009,32 (8):1015−1032. doi: 10.1111/j.1365-3040.2009.01985.x
[19] Chary SN,Hicks GR,Choi YG,Carter D,Raikhel NV. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis[J]. Plant Physiol,2008,146 (1):97−107. doi: 10.1104/pp.107.107441
[20] Suzuki N,Bajad S,Shuman J,Shulaev V,Mittler R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana[J]. J Biol Chem,2008,283 (14):9269−9275. doi: 10.1074/jbc.M709187200
[21] Lin MF,Jia RH,Li JC,Zhang MJ,Chen HB,et al. Evolution and expression patterns of the trehalose-6-phosphate synthase gene family in drumstick tree (Moringa oleifera Lam.)[J]. Planta,2018,248 (4):999−1015. doi: 10.1007/s00425-018-2945-3
[22] Zang BS,Li HW,Li WJ,Deng XW,Wang XP. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice[J]. Plant Mol Biol,2011,76 (6):507−522. doi: 10.1007/s11103-011-9781-1
[23] 蒋伟. 玉米海藻糖-6-磷酸合成酶基因家族的功能验证和差异表达分析[D]. 雅安: 四川农业大学, 2010: 32−34. [24] 谢翎,汪章勋,黄勃. 大豆TPS基因家族全基因组鉴定、分类与表达分析[J]. 中国油料作物学报,2014,36(2):160−167. doi: 10.7505/j.issn.1007-9084.2014.02.004 Xie L,Wang ZX,Huang B. Genome-wide identification classification and expression of TPS family genes in soybean[J]. Chinese Journal of Oil Crop Sciences,2014,36 (2):160−167. doi: 10.7505/j.issn.1007-9084.2014.02.004
[25] Gao YH,Yang XY,Yang X,Zhao TY,An XM,Chen Z. Characterization and expression pattern of the trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase gene families in Populus[J]. Int J Biol Macromol,2021,187:9−23. doi: 10.1016/j.ijbiomac.2021.07.096
[26] Xie DW,Wang XN,Fu LS,Sun J,Zheng W,Li ZF. Identification of the trehalose-6-phosphate synthase gene family in winter wheat and expression analysis under conditions of freezing stress[J]. J Genet,2015,94 (1):55−65. doi: 10.1007/s12041-015-0495-z
[27] Jin QJ,Hu X,Li X,Wang B,Wang YJ,et al. Genome-wide identification and evolution analysis of trehalose-6-phosphate synthase gene family in Nelumbo nucifera[J]. Front Plant Sci,2016,7:1445.
[28] Xu YC,Wang YJ,Mattson N,Yang L,Jin QJ. Genome-wide analysis of the Solanum tuberosum (potato) trehalose-6-phosphate synthase (TPS) gene family:evolution and differential expression during development and stress[J]. BMC Genomics,2017,18 (1):926. doi: 10.1186/s12864-017-4298-x
[29] Hu X,Wu ZD,Luo ZY,Burner DM,Pan YB,Wu CW. Genome-wide analysis of the trehalose-6-phosphate synthase (TPS) gene family and expression profiling of ScTPS genes in sugarcane[J]. Agronomy,2020,10 (7):969. doi: 10.3390/agronomy10070969
[30] Dan YY,Niu Y,Wang CL,Yan M,Liao WB. Genome-wide identification and expression analysis of the trehalose-6-phosphate synthase (TPS) gene family in cucumber (Cucumis sativus L.)[J]. PeerJ,2021,9:e11398. doi: 10.7717/peerj.11398
[31] Li YF,Zhang MF,Zhang M,Jia GX. Analysis of global gene expression profiles during the flowering initiation process of Lilium × formolongi[J]. Plant Mol Biol,2017,94 (4-5):361−379. doi: 10.1007/s11103-017-0612-x
[32] Sun J,Dai ZG,Zhang XY,Tang Q,Cheng CH,et al. Identification of TPS and TPP gene families in Cannabis sativa and their expression under abiotic stresses[J]. Biol Plant,2022,66:14−23. doi: 10.32615/bp.2021.051
[33] Kosmas SA,Argyrokastritis A,Loukas MG,Eliopoulos E,Tsakas S,Kaltsikes PJ. Isolation and characterization of drought-related trehalose 6-phosphate-synthase gene from cultivated cotton (Gossypium hirsutum L.)[J]. Planta,2006,223 (2):329−339. doi: 10.1007/s00425-005-0071-5
[34] Morabito C,Secchi F,Schubert A. Grapevine TPS (trehalose-6-phosphate synthase) family genes are differentially regulated during development,upon sugar treatment and drought stress[J]. Plant Physiol Biochem,2021,164:54−62. doi: 10.1016/j.plaphy.2021.04.032
[35] Yang YJ,Ma KF,Zhang TX,Li LL,Wang J,et al. Characteristics and expression analyses of trehalose-6-phosphate synthase family in Prunus mume reveal genes involved in trehalose biosynthesis and drought response[J]. Biomolecules,2020,10 (10):1358. doi: 10.3390/biom10101358
[36] Yeo ET,Kwon HB,Han SE,Lee JT,Ryu JC,Byu MO. Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae[J]. Mol Cells,2000,10 (3):263−268.
[37] Li HW,Zang BS,Deng XW,Wang XP. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice[J]. Planta,2011,234 (5):1007−1018. doi: 10.1007/s00425-011-1458-0
[38] Han BY,Fu LL,Zhang D,He XQ,Chen Q,et al. Interspecies and intraspecies analysis of trehalose contents and the biosynthesis pathway gene family reveals crucial roles of trehalose in osmotic-stress tolerance in cassava[J]. Int J Mol Sci,2016,17 (7):1077. doi: 10.3390/ijms17071077
[39] Stiller I,Dulai S,Kondrák M,Tarnai R,Szabó L,et al. Effects of drought on water content and photosynthetic parameters in potato plants expressing the trehalose-6-phosphate synthase gene of Saccharomyces cerevisiae[J]. Planta,2008,227 (2):299−308.
[40] Liu YB,Han LZ,Qin LJ,Zhao DG. Saccharomyces cerevisiae gene TPS1 improves drought tolerance in Zea mays L. by increasing the expression of SDD1 and reducing stomatal density[J]. Plant Cell Tissue Organ Cult,2015,120 (2):779−789. doi: 10.1007/s11240-014-0647-5
[41] El-Bashiti T,Hamamcı H,Öktem HA,Yücel M. Biochemical analysis of trehalose and its metabolizing enzymes in wheat under abiotic stress conditions[J]. Plant Sci,2005,169 (1):47−54. doi: 10.1016/j.plantsci.2005.02.024
[42] Song JB,Mao HY,Cheng J,Zhou Y,Chen RR,et al. Identification of the trehalose-6-phosphate synthase gene family in Medicago truncatula and expression analysis under abiotic stresses[J]. Gene,2021,787:145641. doi: 10.1016/j.gene.2021.145641
[43] Almeida AM,Villalobos E,Araújo SS,Leyman B,van Dijck P,et al. Transformation of tobacco with an Arabidopsis thaliana gene involved in trehalose biosynthesis increases tolerance to several abiotic stresses[J]. Euphytica,2005,146 (1-2):165−176. doi: 10.1007/s10681-005-7080-0
[44] Vishal B,Krishnamurthy P,Ramamoorthy R,Kumar PP. OsTPS8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition[J]. New Phytol,2019,221 (3):1369−1386. doi: 10.1111/nph.15464
[45] Jiang T,Zhai H,Wang FB,Zhou HN,Si ZZ,et al. Cloning and characterization of a salt tolerance-associated gene encoding trehalose-6-phosphate synthase in sweetpotato[J]. J Integr Agric,2014,13 (8):1651−1661. doi: 10.1016/S2095-3119(13)60534-1
[46] Yuan GP,Liu JP,An GL,Li WH,Si WJ,et al. Genome-wide identification and characterization of the trehalose-6-phosphate synthetase (TPS) gene family in watermelon (Citrullus lanatus) and their transcriptional responses to salt stress[J]. Int J Mol Sci,2021,23 (1):276. doi: 10.3390/ijms23010276
[47] Miranda JA,Avonce N,Suárez R,Thevelein JM,van Dijck P,Iturriaga G. A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis[J]. Planta,2007,226 (6):1411−1421. doi: 10.1007/s00425-007-0579-y
[48] Lyu JI,Park JH,Kim JK,Bae CH,Jeong WJ,et al. Enhanced tolerance to heat stress in transgenic tomato seeds and seedlings overexpressing a trehalose-6-phosphate synthase/phosphatase fusion gene[J]. Plant Biotechnol Rep,2018,12 (6):399−408. doi: 10.1007/s11816-018-0505-8
[49] Mollavali M,Börnke F. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase genes of tomato (Solanum lycopersicum L.) and analysis of their differential expression in response to temperature[J]. Int J Mol Sci,2022,23 (19):11436. doi: 10.3390/ijms231911436
[50] 庞强强,蔡兴来,孙晓东,张文,周曼. 大白菜TPS基因家族鉴定及其在高温胁迫下的表达分析[J]. 分子植物育种,2020,18(8):2452−2459. Pang QQ,Cai XL,Sun XD,Zhang W,Zhou M. Identification of TPS gene family in Chinese cabbage (Brassica campestris L.) and its expression under heat stress[J]. Molecular Plant Breeding,2020,18 (8):2452−2459.
[51] 谢冬微. 冬小麦低温下基因表达谱分析及海藻糖基因家族研究[D]. 哈尔滨: 东北农业大学, 2014: 77−83. [52] 丁菲,庞磊,李叶云,葛菁,江昌俊. 茶树海藻糖-6-磷酸合成酶基因(CsTPS)的克隆及表达分析[J]. 农业生物技术学报,2012,20(11):1253−1261. Ding F,Pang L,Li YY,Ge J,Jiang CJ. Cloning and expression analysis of trehalose-6-phosphate synthase gene(CsTPS) from tea plant (Camellia sinensis (L. ) O. Kuntz)[J]. Journal of Agricultural Biotechnology,2012,20 (11):1253−1261.
[53] Liu X,Fu LS,Qin P,Sun YL,Liu J,Wang XN. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana[J]. Gene,2019,710:210−217. doi: 10.1016/j.gene.2019.06.006
[54] Avonce N,Leyman B,Mascorro-Gallardo JO,van Dijck P,Thevelein JM,Iturriaga G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose,abscisic acid,and stress signaling[J]. Plant Physiol,2004,136 (3):3649−3659. doi: 10.1104/pp.104.052084
[55] Wei XR,Ling W,Ma YW,Du JL,Cao FX,et al. Genome-wide analysis of the trehalose-6-phosphate synthase gene family in rose (Rosa chinensis) and differential expression under heat stress[J]. Horticulturae,2022,8 (5):429. doi: 10.3390/horticulturae8050429
[56] Zhang Q,Zhang M,Zhao YQ,Hu H,Huang YX,Jia GX. Identification of trehalose-6-phosphate synthase (TPS)-coding genes involved in flowering induction of Lilium × formolongi[J]. Plant Physiol Biochem,2022,171:84−94. doi: 10.1016/j.plaphy.2021.12.025
[57] Lin MF,Ma SY,Quan KH,Yang ED,Hu L,Chen XY. Comparative transcriptome analysis provides insight into the molecular mechanisms of long-day photoperiod in Moringa oleifera[J]. Physiol Mol Biol Plants,2022,28 (5):935−946. doi: 10.1007/s12298-022-01186-4
[58] 赵美丽,倪军,陈茂盛,徐增富. 超量表达小桐子JcTPS1促进拟南芥开花并增加叶片花青素含量[J]. 分子植物育种,2018,16(1):255−261. Zhao ML,Ni J,Chen MS,Xu ZF. Over-expression of JcTPS1 from Jatropha curcas induces early flowering of Arabidopsis and improves anthocyanin accumulation in leaves[J]. Molecular Plant Breeding,2018,16 (1):255−261.
[59] 陈宏艳,李小二,李忠光. 糖信号及其在植物响应逆境胁迫中的作用[J]. 生物技术通报,2022,38(7):80−89. Chen HY,Li XE,Li ZG. Sugar signaling and its role in plant response to environmental stress[J]. Biotechnology Bulletin,2022,38 (7):80−89.
[60] Bischof S. Life is sweeter with trehalose 6-phosphate[J]. Plant Cell,2020,32 (6):1784−1785. doi: 10.1105/tpc.20.00276
[61] Paul MJ,Gonzalez-Uriarte A,Griffiths CA,Hassani-Pak K. The role of trehalose 6-phosphate in crop yield and resilience[J]. Plant Physiol,2018,177 (1):12−23. doi: 10.1104/pp.17.01634
[62] Saidi A,Hajibarat Z. Computational analysis and expression study of trehalose 6-phosphate synthase (TPS) in rice (Oryza sativa)[J]. Sci Study Res:Chem Chem Eng,Biotechnol,Food Ind,2021,22 (1):13−25.
[63] 项阳,刘延波,赵德刚,Yi Li. 转TPS1基因促进干旱胁迫条件下的花青素积累提高玉米植株抗旱性[J]. 植物生理学报,2015,51(11):2054−2062. Xiang Y,Liu YB,Zhao DG,Yi L. Improve drought tolerance via accumulating anthocyanidin under drought stress in TPS1 transgenic maize[J]. Plant Physiology Journal,2015,51 (11):2054−2062.
[64] Krishnamurthy P,Jyothi-Prakash PA,Qin L,He J,Lin QS,et al. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis[J]. Plant Cell Environ,2014,37 (7):1656−1671. doi: 10.1111/pce.12272
[65] Gabriel C,Fernhout JJ,Fichtner F,Feil R,Lunn JE,et al. Genetic manipulation of trehalose-6-phosphate synthase results in changes in the soluble sugar profile in transgenic sugarcane stems[J]. Plant Direct,2021,5 (11):e358.
[66] Romero C,Bellés JM,Vayá JL,Serrano R,Culiáñez-Macià FA. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants:pleiotropic phenotypes include drought tolerance[J]. Planta,1997,201 (3):293−297. doi: 10.1007/s004250050069
[67] Garg AK,Kim JK,Owens TG,Ranwala AP,Choi YD,et al. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses[J]. Proc Natl Acad Sci USA,2002,99 (25):15898−15903. doi: 10.1073/pnas.252637799
[68] 杜丽璞,徐惠君,叶兴国,林忠平. 小麦转TPS基因植株的获得及其初步功能鉴定[J]. 麦类作物学报,2007,27(3):369−373. doi: 10.3969/j.issn.1009-1041.2007.03.001 Du LP,Xu HJ,Ye XG,Lin ZP. Transgenic wheat plants with trehalose-6-phosphate synthase (TPS) gene and identification of their function[J]. Journal of Triticeae Crops,2007,27 (3):369−373. doi: 10.3969/j.issn.1009-1041.2007.03.001
[69] 程庚. 垫状卷柏海藻糖-6-磷酸合成酶基因转化玉米[D]. 雅安: 四川农业大学, 2012: 31−37.




 下载:
下载: